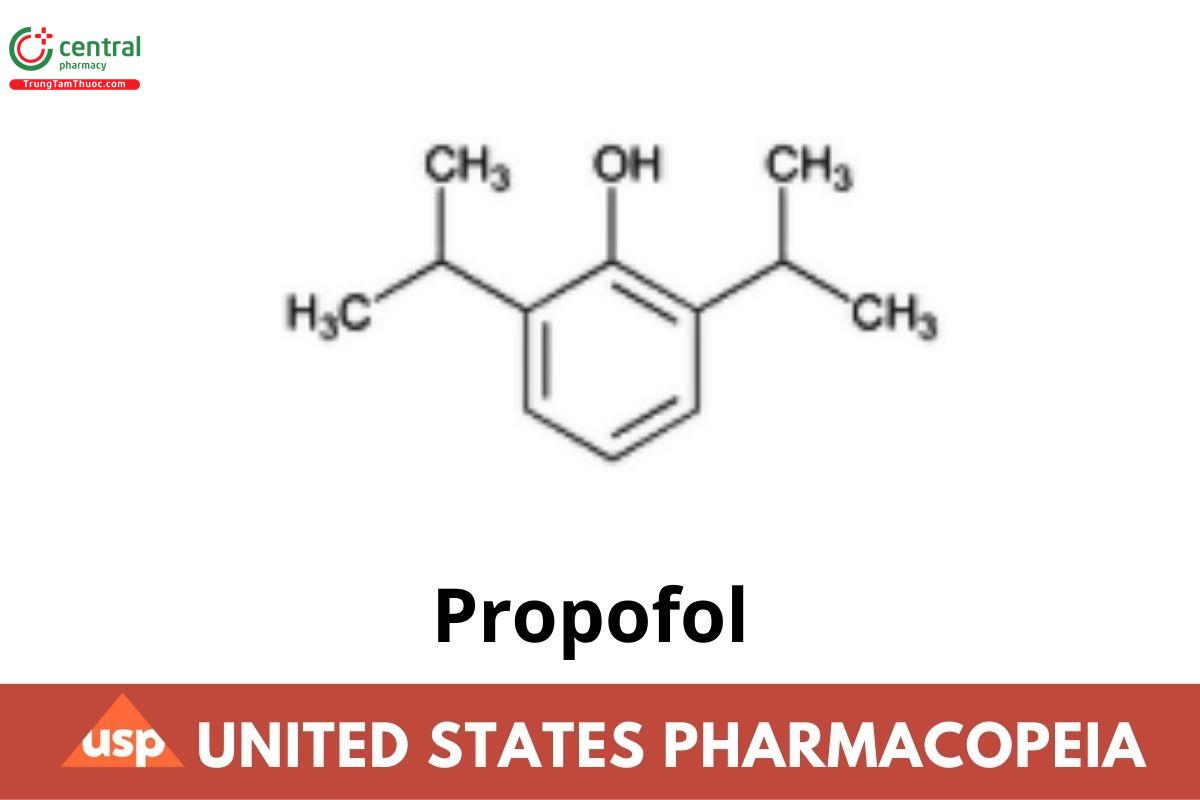
Propofol
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Propofol contains NLT 98.0% and NMT 102.0% of propofol (C₁₂H₁₈O).
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197F
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay, Procedure 1 or Procedure 2.
3 ASSAY
Procedure 1
[Note—This is to be performed in conjunction with Organic Impurities, Procedure 1.]
Internal standard solution: 10 mg/mL of USP 2,4,6-Tritertbutylphenol RS in methanol
Standard solution: 10 mg/mL of USP Propofol RS in Internal standard solution
Sample solution: 10 mg/mL of Propofol in Internal standard solution
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.53-mm × 30-m; coated with a 1.2-µm phase G16
Temperatures
Detector: 300°
Injection port: 250°
Column: See Table 1
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
|---|---|---|---|
| 145 | — | 145 | 20 |
| 145 | 5 | 200 | 5 |
Carrier gas: Helium
Flow rate: 8 mL/min
Injection volume: 1.0 µL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.5
Relative standard deviation: NMT 1.5% for the peak response ratio of propofol to the internal standard for five replicate injections
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of propofol (C₁₂H₁₈O) in the portion of Propofol taken:
Result = (Rᵤ / Rₛ) × (Cₛ / Cᵤ) × 100
Rᵤ = peak response ratio of propofol to the internal standard from the Sample solution
Rₛ = peak response ratio of propofol to the internal standard from the Standard solution
Cₛ = concentration of USP Propofol RS in the Standard solution (mg/mL)
Cᵤ = concentration of Propofol in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0%
Change to read:
Procedure 2
[Note—This is to be performed in conjunction with Organic Impurities, Procedure 2.]
Mobile phase: Hexane, acetonitrile, and ▲alcohol, absolute▲ (ERR 1-Mar-2024) (990:7.5:1)
Standard solution: 2.4 mg/mL of USP Propofol RS in hexane
Sample solution: 2.4 mg/mL of Propofol in hexane
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 275 nm
Column: 4.6-mm × 20-cm; 5-µm packing L3
Flow rate: 2 mL/min
Injection volume: 10 µL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 1.5
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of propofol (C₁₂H₁₈O) in the portion of Propofol taken:
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × 100
rᵤ = peak response of propofol from the Sample solution
rₛ = peak response of propofol from the Standard solution
Cₛ = concentration of USP Propofol RS in the Standard solution (mg/mL)
Cᵤ = concentration of Propofol in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0%
4 IMPURITIES
Organic Impurities, Procedure 1
[Note—On the basis of knowledge of the manufacturing process, either (1) Organic Impurities, Procedure 1 is performed in conjunction with Limit of Propofol Related Compound A, Limit of Propofol Related Compound B, Procedure 1, and Assay, Procedure 1; or (2) Organic Impurities, Procedure 2 is performed in conjunction with Limit of Propofol Related Compound B, Procedure 2 and the Assay, Procedure 2.]
System suitability solution: 100 mg/mL of USP Propofol Resolution Mixture RS in methanol
Standard solution: 0.1 mg/mL of USP Propofol RS in methanol
Sample solution: 100 mg/mL of Propofol in methanol
Chromatographic system: Proceed as directed in the Assay, Procedure 1.
System suitability
Samples: System suitability solution and Standard solution
[Note—See Table 2 for the relative retention times.]
Suitability requirements
Resolution: NLT 2 between propofol and 2-isopropyl-6-n-propylphenol, System suitability solution
Relative standard deviation: NMT 3.5% for six replicate injections, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Propofol taken:
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × 100
rᵤ = peak response for each impurity from the Sample solution
rₛ = peak response for propofol from the Standard solution
Cₛ = concentration of USP Propofol RS in the Standard solution (mg/mL)
Cᵤ = concentration of Propofol in the Sample solution (mg/mL)
Acceptance criteria: See Table 2.
Table 2
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
|---|---|---|
| Propofol related compound C | 0.18 | 0.1 |
| Propofol | 1.0 | — |
| 2-Isopropyl-6-n-propylphenol | 1.1 | 0.1 |
| Any unspecified impurity | — | 0.1 |
| Total impurities | — | 0.3 |
Organic Impurities, Procedure 2
Mobile phase and Chromatographic system: Proceed as directed in the Assay, Procedure 2.
System suitability solution: 0.1 µL/mL of USP Propofol RS and 0.3 µL/mL of USP Propofol Related Compound B RS in hexane
Peak identification solution: 0.25 mg/mL of USP Propofol Related Compound A RS, 100 µL/mL of the Propofol that is under test, and 5 µL/mL of USP Propofol Related Compound C RS in hexane
Sample solution: 100 mg/mL of Propofol in hexane
Reference solution: 0.1 mg/mL of Propofol in hexane from the Sample solution
System suitability
Samples: System suitability solution and Peak identification solution
[Note—See Table 3 for the relative retention times.]
Suitability requirements
Resolution: NLT 4.0 between propofol related compound B and propofol, System suitability solution
Analysis
Samples: Sample solution and Reference solution
Calculate the percentage of each impurity in the portion of Propofol taken:
Result = (rᵤ / rₛ) × (1/F) × D × 100
rᵤ = peak response of each impurity from the Sample solution
rₛ = peak response of propofol from the Reference solution
F = relative response factor (see Table 3)
D = dilution factor used to prepare the Reference solution, 0.001
Acceptance criteria: See Table 3.
Table 3
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Propofol related compound C | 0.5 | 0.2 | 0.2 |
| Propofol | 1.0 | — | — |
| Propofol related compound A | 5.0 | 4.0 | 0.01 |
| Any unspecified impurity | — | 1.0 | 0.05 |
| Total impurities | — | — | 0.3 |
Limit of Propofol Related Compound A
[Note—This test is to be performed in conjunction with Organic Impurities, Procedure 1.]
Mobile phase: Acetonitrile, methanol, and water (50:10:40)
Standard solution: 20 µg/mL of USP Propofol Related Compound A RS in methanol
Sample solution: 20 mg/mL of Propofol in methanol
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 270 nm
Column: 4.6-mm × 15-cm; packing L1
Flow rate: 1.5 mL/min
Injection volume: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Column efficiency: NLT 6000 theoretical plates
Relative standard deviation: NMT 15% for six replicate injections
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of propofol related compound A in the portion of Propofol taken:
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × 100
rᵤ = peak response of propofol related compound A from the Sample solution
rₛ = peak response of propofol related compound A from the Standard solution
Cₛ = concentration of USP Propofol Related Compound A RS in the Standard solution (mg/mL)
Cᵤ = concentration of Propofol in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.1% of propofol related compound A
Limit of Propofol Related Compound B, Procedure 1
Sample: Propofol
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy 〈857〉.)
Mode: UV
Analytical wavelength: 330 nm
Blank: Air
Analysis: Measure the absorbance of the Sample using air as the blank.
Acceptance criteria: NMT 0.1%; the absorbance of the Sample is NMT 0.4 absorbance units.
Limit of Propofol Related Compound B, Procedure 2
[Note—This is to be performed in conjunction with Organic Impurities, Procedure 2.]
Mobile phase: Prepare as directed in the Assay, Procedure 2.
Standard solution: 5 µg/mL of USP Propofol Related Compound B RS in hexane
Sample solution: 50 mg/mL of Propofol in hexane
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 20-cm; 5-µm packing L3
Flow rate: 2 mL/min
Injection volume: 20 µL
Analysis
Samples: Standard solution and Sample solution
[Note—The relative retention times for propofol related compound B and propofol are about 0.8 and 1.0, respectively.]
Calculate the percentage of propofol related compound B in the portion of Propofol taken:
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × 100
rᵤ = peak response of propofol related compound B from the Sample solution
rₛ = peak response of propofol related compound B from the Standard solution
Cₛ = concentration of USP Propofol Related Compound B RS in the Standard solution (mg/mL)
Cᵤ = concentration of Propofol in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.05% of propofol related compound B
5 SPECIFIC TESTS
Refractive Index 〈831〉: 1.5125–1.5145 at 20°
ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers under an atmosphere of inert gas, and protect from light. Store at controlled room temperature.
Labeling: The labeling indicates the Organic Impurities procedure with which the article complies if a procedure other than Organic Impurities, Procedure 1 is used.
USP Reference Standards 〈11〉
USP Propofol RS
USP Propofol Related Compound A RS
3,3′-5,5′-Tetraisopropyldiphenol;
Also known as 3,3′,5,5′-Tetraisopropylbiphenyl-4,4′-diol.
C₂₄H₃₄O₂ 354.53
USP Propofol Related Compound B RS
2,6-Diisopropyl-1,4-benzoquinone;
Also known as 2,6-Diisopropylcyclohexa-2,5-diene-1,4-dione.
C₁₂H₁₆O₂ 192.26
USP Propofol Related Compound C RS
2,6-Diisopropylphenyl isopropyl ether;
Also known as 2-Isopropoxy-1,3-diisopropylbenzene.
C₁₅H₂₄O 220.36
USP Propofol Resolution Mixture RS
Contains a mixture of the following two compounds:
Propofol.
2-Isopropyl-6-n-propylphenol.
USP 2,4,6-Tritertbutylphenol RS
2,4,6-Tri-tert-butylphenol.
C₁₈H₃₀O 262.44


