Procyclidine Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
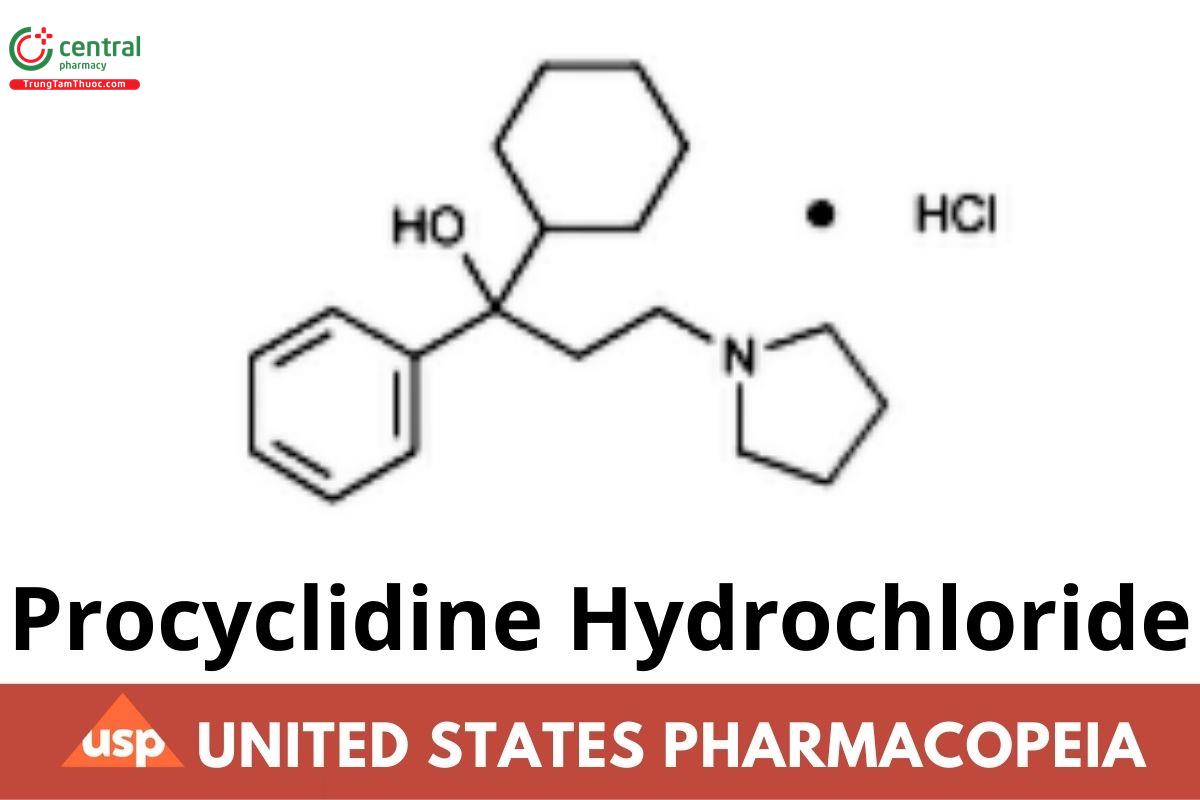
C19H29NO .HCl 323.90
1-Pyrrolidinepropanol, α-cyclohexyl-α-phenyl-, hydrochloride.
α-Cyclohexyl-α-phenyl-1-pyrrolidinepropanol hydrochloride CAS RN®: 1508-76-5; UNII: CQC932Z7YW.
Procyclidine Hydrochloride contains not less than 99.0 percent and not more than 101.0 percent of C19H29NO .HCl, calculated on the dried basis.
Packaging and storage—Preserve in tight, light-resistant containers, and store in a dry place.
USP Reference standards 〈11〉—
USP Procyclidine Hydrochloride RS
Identification—
Change to read:
A: Spectroscopic Identication Tests 〈197〉, Infrared Spectroscopy: 197K.
B: Dissolve about 250 mg in 10 mL of water in a separator, render alkaline with 6 N ammonium hydroxide, and extract with three 10-mL portions of ether. Filter the ether extracts slowly through a layer of about 2 g of anhydrous granular sodium sulfate supported on glass wool, evaporate the ether with a current of warm air, and scratch the surface of the container to induce crystallization of the residue: the procyclidine so obtained melts between 83° and 87°, the procedure for Class I being used (see Melting Range or Temperature 〈741〉). C: A solution (1 in 100) responds to the tests for Chloride 〈191〉. pH 〈791〉: between 5.0 and 6.5, in a solution (1 in 100).
Loss on drying 〈731〉—Dry it in vacuum at 105° for 4 hours: it loses not more than 0.5% of its weight.
Residue on ignition 〈281〉: not more than 0.1%.
Related compounds—Dissolve approximately 200 mg of Procyclidine Hydrochloride in 20 mL of water, and render the solution alkaline by adding 1.5 mL of 6 N ammonium hydroxide. Extract with three 15-mL portions of chloroform, wash the combined extracts with 20 mL of water, discard the water washing, and filter the chloroform solution through a layer of 3 to 4 g of anhydrous granular sodium sulfate supported on glass wool. Reduce the volume to 5 mL by evaporating with the aid of gentle heat and a current of air. Inject 2 µL of this solution into a suitable gas chromatograph (see Chromatography 〈621〉) equipped with a ame-ionization detector, and record the chromatogram to 2.5 relative to the retention time of the principal (procyclidine) peak. Under typical conditions, the instrument contains a 1-m × 2-mm glass column packed with 10% polyethylene glycol 20,000 and 2% potassium hydroxide on packing S1A. The column is maintained at a temperature of about 180°, the injection port is maintained at 210°, the detector block is maintained at about 220°, and dry helium is used as the carrier gas at a flow rate of about 60 mL per minute. From the total area under the curve, excluding the solvent peak, calculate the percentage of total impurities by area normalization: not more than 4.0% is found.
Assay—Dissolve about 700 mg of Procyclidine Hydrochloride, accurately weighed, in 75 mL of glacial acetic acid in a 250-mL beaker, warming, if necessary, to effect solution. Cool, add 10 mL of mercuric acetate TS, and titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 32.39 mg of C19H29NO .HCl.


