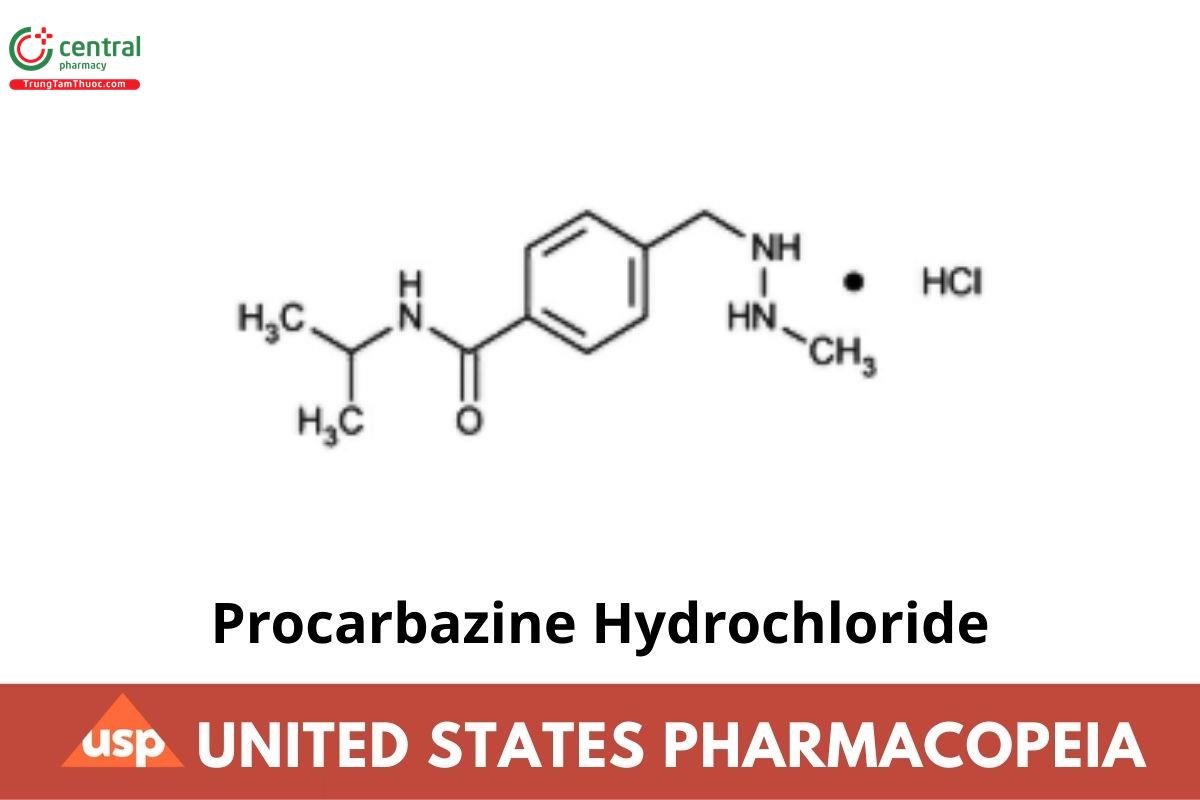
Procarbazine Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
» Procarbazine Hydrochloride contains not less than 98.5 percent and not more than 100.5 percent of C₁₂H₁₉N₃O · HCl.
[Caution—Handle Procarbazine Hydrochloride with exceptional care, since it is a highly potent agent.]
Packaging and storage—Preserve in tight, light-resistant containers.
USP Reference standards 〈11〉—
USP Procarbazine Hydrochloride RS
1 Identification—
Change to read:
A: ▲Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M▲ (CN 1-May-2020).
Change to read:
B: ▲Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U▲ (CN 1-May-2020) —
[Note—Use low-actinic glassware throughout this test.]
Solution: 10 µg per mL.
Medium: 0.1 N hydrochloric acid.
Absorptivities at 232 nm do not differ by more than 3.0%.
Residue on ignition 〈281〉: not more than 0.1%.
2 Assay—
Dissolve about 0.75 g of Procarbazine Hydrochloride, accurately weighed, in 100 mL of water. Titrate with 0.1 N sodium hydroxide VS, determining the endpoint potentiometrically, using a calomel-glass electrode system. Each mL of 0.1 N sodium hydroxide is equivalent to 25.78 mg of C₁₂H₁₉N₃O · HCl.


