Prednisone
If you find any inaccurate information, please let us know by providing your feedback here
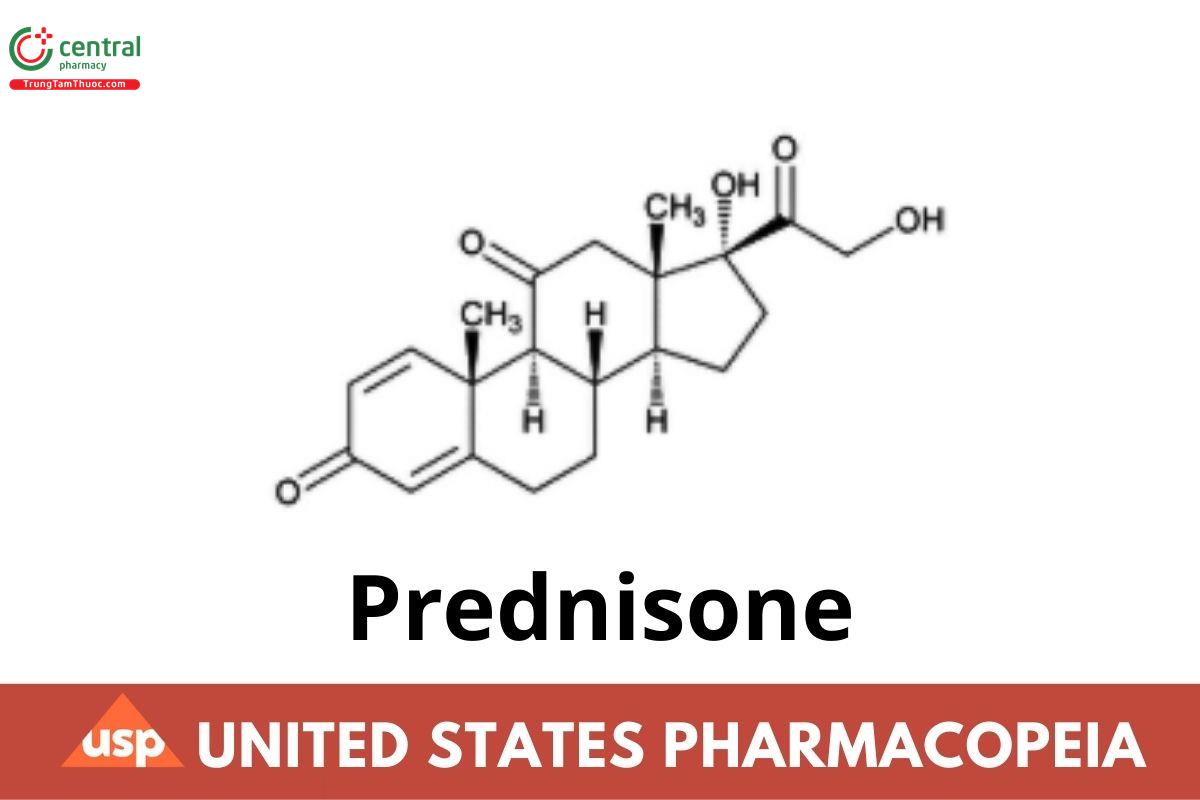
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Prednisone contains NLT 97.0% and NMT 102.0% of prednisone (C₂₁H₂₆O₅), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. ▲Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K▲ (CN 1-May-2020)
Analysis: If a difference appears, dissolve portions of both the sample and the Reference Standard in methanol, evaporate the solutions to dryness, and repeat the tests.
Acceptance criteria: Meets the requirements
B.
Analysis 1: Dissolve 6 mg in 2 mL of sulfuric acid, and allow to stand for 5 min.
Acceptance criteria 1: An orange color is produced.
Analysis 2: Pour the resulting solution from Analysis 1 into 10 mL of water.
Acceptance criteria 2: The color changes first to yellow and then, gradually, to bluish green.
3 ASSAY
Procedure
Mobile phase: Peroxide-free tetrahydrofuran, methanol, and water (250:62:688)
Diluent: Methanol and water (1:1)
Standard stock solution: 0.2 mg/mL of USP Prednisone RS in Diluent
Internal standard solution: 110 µg/mL of acetanilide in Diluent
Standard solution: 20 µg/mL of USP Prednisone RS and 11 µg/mL of acetanilide in Diluent from the Standard stock solution and the Internal standard solution, respectively. Prepare this solution fresh.
Sample stock solution: 0.2 mg/mL of Prednisone in Diluent
Sample solution: 20 µg/mL of Prednisone and 11 µg/mL of acetanilide in Diluent from the Sample stock solution and the Internal standard solution, respectively. Prepare this solution fresh.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4-mm × 25-cm; packing L1
Flow rate: 1.0 mL/min
Injection volume: 10 µL
[Note—The retention times of acetanilide and prednisone are about 6 and 8 min, respectively.]
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 3 between prednisone and acetanilide
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of prednisone (C₂₁H₂₆O₅) in the portion of Prednisone taken:
Result = (Rᵤ / Rₛ) × (Cₛ / Cᵤ) × 100
Rᵤ = peak response ratio of prednisone to acetanilide from the Sample solution
Rₛ = peak response ratio of prednisone to acetanilide from the Standard solution
Cₛ = concentration of USP Prednisone RS in the Standard solution (µg/mL)
Cᵤ = concentration of Prednisone in the Sample solution (µg/mL)
Acceptance criteria: 97.0%–102.0% on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉
Sample: 100 mg
Acceptance criteria: Negligible
Organic Impurities
Mobile phase: Chloroform and methanol (98:2)
Sample solution: 1.25 mg/mL of Prednisone in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 6.0-mm × 4.0-cm; packing L3
Flow rate: 1 mL/min
Injection volume: 5 µL
System suitability
Sample: Sample solution
Suitability requirements
Column efficiency: NLT 2500 theoretical plates
Relative standard deviation: NMT 2.0%
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Prednisone taken:
Result = (rᵢ / rₜ) × 100
rᵢ = peak response for each impurity
rₜ = sum of all the peak responses
Acceptance criteria
Any individual impurity: NMT 1.5%
Total impurities: NMT 2.0%
5 SPECIFIC TESTS
Optical Rotation, Specific Rotation 〈781S〉
Sample solution: 5 mg/mL in dioxane
Acceptance criteria: +167° to +175°
Water Determination, Method I 〈921〉
Acceptance criteria: NMT 1.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers, protected from light and moisture. Store at controlled room temperature.
USP Reference Standards 〈11〉
USP Prednisone RS


