Prazosin Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
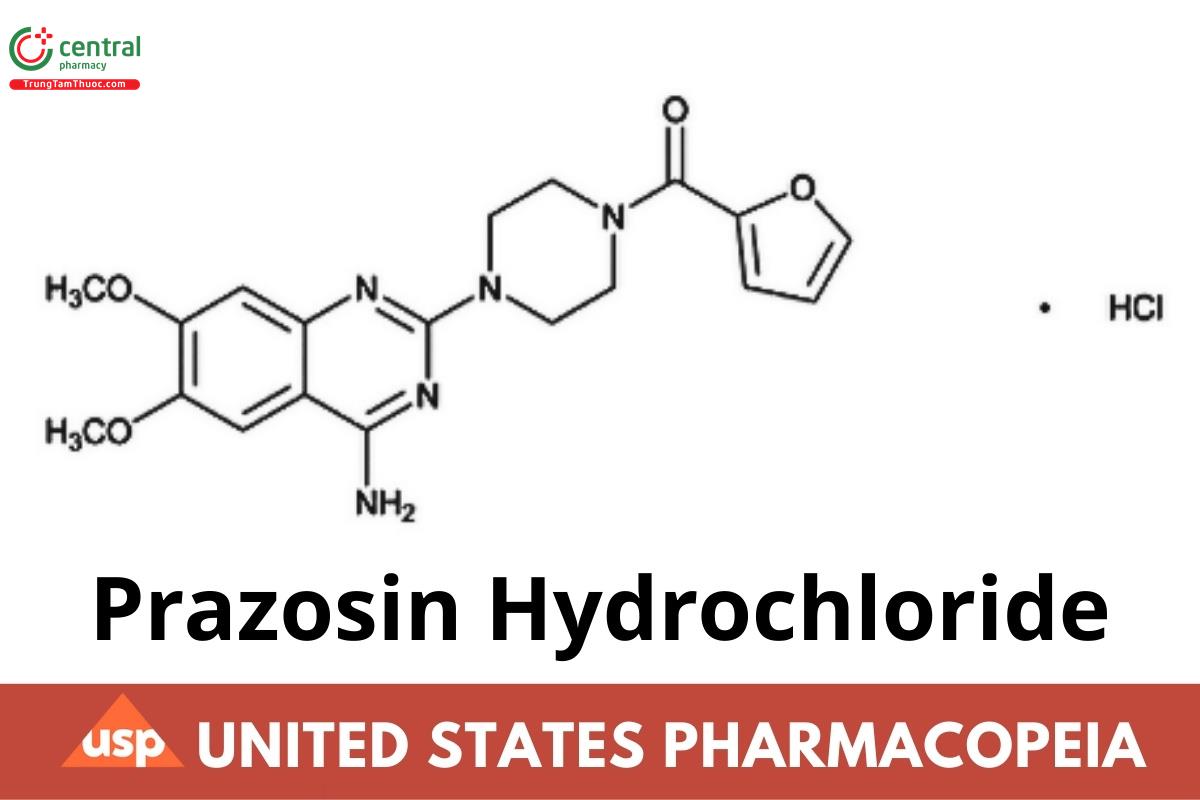
C19H21N5O4 . HCl 419.86
Piperazine, 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)-, monohydrochloride;
1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furoyl)piperazine monohydrochloride CAS RN®: 19237-84-4; UNII: X0Z7454B90.
1 DEFINITION
Prazosin Hydrochloride contains NLT 97.0% and NMT 103.0% of prazosin hydrochloride (C19H21N5O4 . HCl), calculated on the anhydrous basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K or 197A
B. The UV spectrum of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
C. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
D. Identification Tests—General 〈191〉, Chemical Identification Tests, Chloride: Meets the requirements
3 ASSAY
3.1 Procedure
[Caution—Care should be taken to prevent inhaling particles of Prazosin Hydrochloride and to prevent its contacting any part of the body.]
Mobile phase: Methanol, glacial acetic acid, and water (700:10:300). Add 0.2 mL of diethylamine to 1 L of Mobile phase such that the retention time of prazosin hydrochloride is 6–10 min.
Diluent: Methanol and water (70:30)
Standard stock solution: 1 mg/mL of USP Prazosin Hydrochloride RS in methanol
Standard solution: 30 µg/mL of USP Prazosin Hydrochloride RS in Diluent from the Standard stock solution
Sample stock solution: 1 mg/mL of Prazosin Hydrochloride in methanol
Sample solution: 30 µg/mL of Prazosin Hydrochloride in Diluent from Sample stock solution
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm. For Identification B, use a diode array detector in the range of 200–400 nm.
Column: 4.6-mm × 25-cm; 5-µm packing L3
Flow rate: Adjusted to obtain a retention time of 6–10 min for prazosin
Injection volume: 5 µL
Run time: NLT 2 times the retention time of the prazosin peak
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 1.10%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of prazosin hydrochloride (C19H21N5O4 . HCl) in the portion of Prazosin Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of prazosin from the Sample solution
rS = peak response of prazosin from the Standard solution
CS = concentration of USP Prazosin Hydrochloride RS in the Standard solution (µg/mL)
CU = concentration of Prazosin Hydrochloride in the Sample solution (µg/mL)
Acceptance criteria: 97.0%–103.0% calculated on the anhydrous basis
4 IMPURITIES
4.1 Residue on Ignition 〈281〉
Sample: 1 g of Prazosin Hydrochloride
Acceptance criteria: NMT 0.4%
[Note—Save the residue for the test for Iron.]
4.2 Iron
Standard stock solution: Dissolve 100 mg of iron wire in 10 mL of hydrochloric acid with the aid of boiling. Cool, transfer to a 1000-mL volumetric flask, and dilute with water to volume.
Standard solution: 4.0 µg/mL of iron in 0.2 N nitric acid from Standard stock solution
Sample solution: Dissolve the residue obtained in the test for Residue on Ignition in 20 mL of 2 N nitric acid. Slowly evaporate this solution to approximately 5 mL; transfer to a 25-mL volumetric flask, using 0.2 N nitric acid as a wash solvent; and dilute with 0.2 N nitric acid to volume.
Instrumental conditions
(See Atomic Absorption Spectroscopy 〈852〉.)
Mode: Atomic absorption
Analytical wavelength: 248 nm
Lamp: Iron hollow-cathode
Flame: Air–acetylene
Blank: Water
Analysis: Determine the absorbances of the Standard solution and the Sample solution against the Blank.
Acceptance criteria: The absorbance of the Sample solution is NMT that of the Standard solution (0.010%).
4.3 Nickel
Standard stock solution: Dissolve 100 mg of nickel in 10 mL of nitric acid with the aid of boiling. Cool, transfer to a 1000-mL volumetric flask, and dilute with water to volume.
Standard solution: 4.0 µg/mL of nickel in 0.2 N nitric acid from Standard stock solution
Sample solution: Prepare as directed in the test for Iron.
Instrumental conditions
(See Atomic Absorption Spectroscopy 〈852〉.)
Mode: Atomic absorption
Analytical wavelength: 232 nm
Lamp: Nickel hollow-cathode
Flame: Air–acetylene
Blank: Water
Analysis: Determine the absorbances of the Standard solution and the Sample solution against the Blank.
Acceptance criteria: The absorbance of the Sample solution is NMT that of the Standard solution (0.010%).
Change to read:
4.4 Organic Impurities
Buffer: 3.48 g/L of sodium pentanesulfonate and 3.64 g/L of tetramethylammonium hydroxide. Adjust with glacial acetic acid to a pH of 5.0.
Mobile phase: Methanol and Buffer (500:500)
System suitability solution: 0.032 mg/mL of USP Metoclopramide Hydrochloride RS and 0.004 mg/mL of USP Prazosin Hydrochloride RS in Mobile phase
Standard solution: 1 µg/mL of USP Prazosin Hydrochloride RS in Mobile phase
Sensitivity solution: 0.5 (ERR 1-Jan-2022) µg/mL of USP Prazosin Hydrochloride RS in Mobile phase from the Standard solution
Sample solution: 1 mg/mL of Prazosin Hydrochloride in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Flow rate: 1 mL/min
Injection volume: 20 µL
Run time: NLT 6 times the retention time of the prazosin peak
System suitability
Samples: System suitability solution, Standard solution, and Sensitivity solution
[Note—The relative retention times of metoclopramide and prazosin are 0.55 and 1.00, respectively.]
Suitability requirements
Resolution: NLT 8 between the metoclopramide and prazosin peaks, System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each individual impurity in the portion of Prazosin Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of each individual impurity from the Sample solution
rS = peak response of prazosin from the Standard solution
CS = concentration of USP Prazosin Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Prazosin Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria
Each individual impurity: NMT 0.2%
Total impurities: NMT 0.5%.
The reporting threshold is 0.05%.
5 SPECIFIC TESTS
Water Determination 〈921〉, Method I
Anhydrous form: NMT 2.0%
Polyhydrate form: 8.0%–15.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers.
Labeling: Label it to indicate whether it is anhydrous or is the polyhydrate.
USP Reference Standards 〈11〉
USP Metoclopramide Hydrochloride RS
USP Prazosin Hydrochloride RS


