Pravastatin Sodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
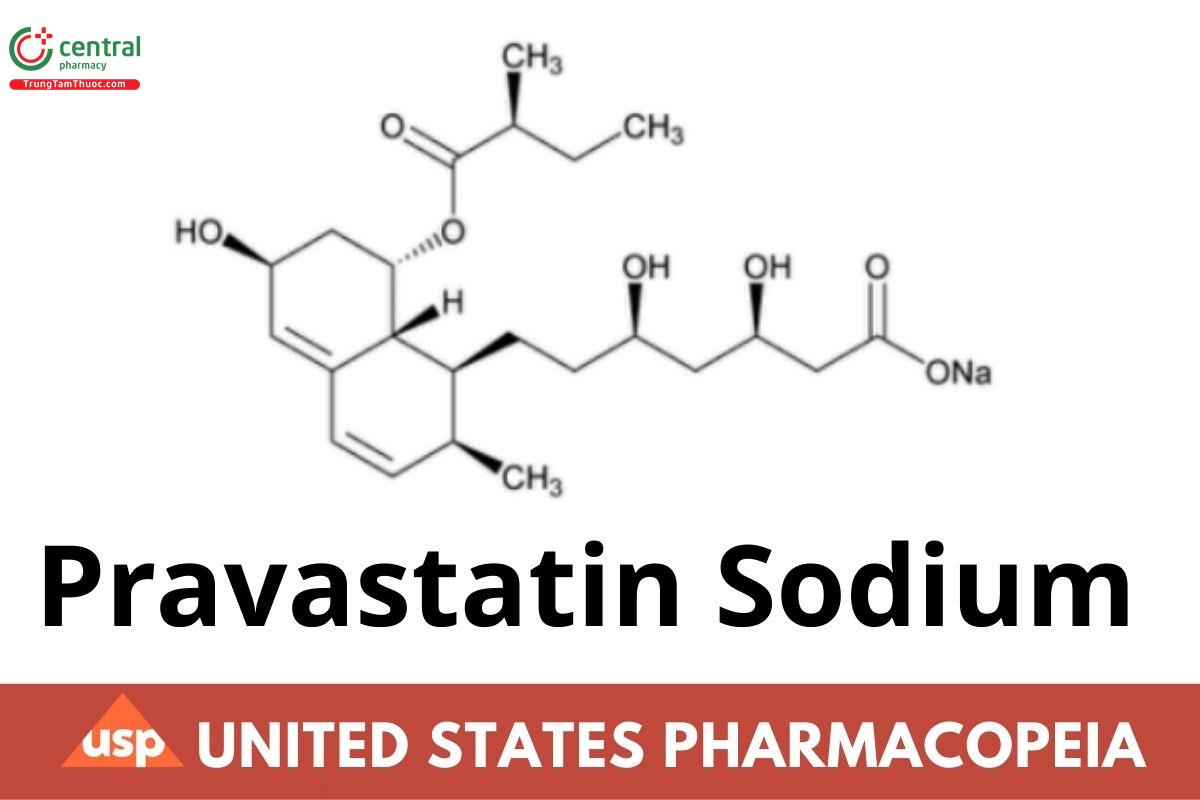
C23H35NaO7 446.52
1-Naphthaleneheptanoic acid, 1,2,6,7,8,8a-hexahydro-β,δ,6-trihydroxy-2-methyl-8-(2-methyl-1-oxobutoxy)-, monosodium salt, [1S-
[1α(βS*,δS*),2α,6α,8β(R*),8aα]]-;
Sodium (βR,δR,1S,2S,6S,8S,8aR)-1,2,6,7,8,8a-hexahydro-β,δ,6,8-tetrahydroxy-2-methyl-1-naphthaleneheptanoate, 8-[(2S)-2-methylbutyrate] CAS RN®: 81131-70-6; UNII: 3M8608UQ61.
DEFINITION
Pravastatin Sodium contains NLT 97.5% and NMT 102.0% of pravastatin sodium (C23H35NaO7), calculated on the anhydrous and solvent-free basis.
1 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. Identification Tests—General 〈191〉, Chemical Identification Tests, Sodium: Meets the requirements of test A • C. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
2 ASSAY
Procedure
Solution A: 0.08 M phosphoric acid solution. Adjust with a 25% sodium hydroxide solution to a pH of 5.5.
Solution B: Acetonitrile and Solution A (20:80)
Solution C: Acetonitrile
Mobile phase: See Table 1.
Table 1
Time (min) | Solution B (%) | Solution C (%) |
0 | 100 | 0 |
7.0 | 90 | 10 |
10.0 | 62.5 | 37.5 |
17.0 | 62.5 | 37.5 |
17.1 | 100 | 0 |
20.0 | 100 | 0 |
System suitability solution: 0.25 mg/mL of USP Pravastatin 1,1,3,3-Tetramethylbutylamine RS and 0.001 mg/mL of USP Pravastatin Related Compound A RS in methanol
Standard solution: 0.25 mg/mL of USP Pravastatin 1,1,3,3-Tetramethylbutylamine RS in methanol
Sample solution: 0.2 mg/mL of Pravastatin Sodium in methanol
2.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 238 nm
Column: 4.0-mm × 10-cm; 3-µm packing L1
Flow rate: 1 mL/min
Injection volume: 10 µL
2.2 System suitability
Sample: System suitability solution
[Note—The relative retention times for pravastatin and pravastatin related compound A are about 1.0 and 1.2, respectively.] Suitability requirements
Resolution: NLT 1.2 between pravastatin and pravastatin related compound A
Relative standard deviation: NMT 2.0% for the pravastatin peak
2.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of pravastatin sodium (C23H35NaO7) in the portion of Pravastatin Sodium taken:
Result = (rU /rS ) × (CS /CU ) × (Mr1 /Mr2 ) × 100
rU = peak response of pravastatin from the Sample solution
rS = peak response of pravastatin from the Standard solution
CS = concentration of USP Pravastatin 1,1,3,3-Tetramethylbutylamine RS in the Standard solution (mg/mL)
CU = concentration of Pravastatin Sodium in the Sample solution (mg/mL)
Mr1 = molecular weight of pravastatin sodium, 446.52
Mr2 = molecular weight of pravastatin 1,1,3,3-tetramethylbutylamine, 553.78
Acceptance criteria: 97.5%–102.0% on the anhydrous and solvent-free basis
3 IMPURITIES
Organic Impurities
[Note—The Standard solution and the Sample solution are maintained at 15° until injected into the chromatograph.] Diluent: Methanol and water (50:50)
Solution A: 0.08 M phosphoric acid solution. Adjust with triethylamine to a pH of 7.0.
Solution B: Acetonitrile, Solution A, and water (18:30:52)
Solution C: Acetonitrile, Solution A, and water (60:30:10)
Mobile phase: See Table 2.
Table 2
Time (min) | Solution B (%) | Solution C (%) |
0 | 100 | 0 |
3.0 | 100 | 0 |
26.5 | 0 | 100 |
26.6 | 100 | 0 |
30.0 | 100 | 0 |
System suitability solution: 0.6 mg/mL of USP Pravastatin 1,1,3,3-Tetramethylbutylamine RS and 0.001 mg/mL of USP Pravastatin Related Compound A RS in Diluent. [Note—USP Pravastatin Related Compound A RS is a sodium salt of 3α-hydroxyisocompactin acid.] Standard solution: 1.25 µg/mL of USP Pravastatin 1,1,3,3-Tetramethylbutylamine RS in Diluent
Sample solution: 0.5 mg/mL of Pravastatin Sodium in Diluent
3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 238 nm
Column: 4.6-mm × 7.5-cm; 3.5-µm packing L1.
[Note—Alternatively, a 4.0-mm × 10-cm; 3-µm packing L1 can be used.]
Flow rate: 1 mL/min
Injection volume: 10 µL
3.2 System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for pravastatin and pravastatin related compound A are about 1.0 and 1.1, respectively.] Suitability requirements
Resolution: NLT 2.0 between pravastatin and pravastatin related compound A, System suitability solution
Relative standard deviation: NMT 10.0%, Standard solution
3.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each specified or any unspecified impurity in the portion of Pravastatin Sodium taken:
Result = (rU /rS ) × (CS /CU ) × (Mr1 /Mr2 ) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of pravastatin from the Standard solution
CS = concentration of USP Pravastatin 1,1,3,3-Tetramethylbutylamine RS in the Standard solution (mg/mL)
CU = concentration of Pravastatin Sodium in the Sample solution (mg/mL)
Mr1 = molecular weight of pravastatin sodium, 446.52
Mr2 = molecular weight of pravastatin 1,1,3,3-tetramethylbutylamine, 553.78
Acceptance criteria: See Table 3. The reporting level for impurities is 0.05%.
Table 3
Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
3″-Hydroxypravastatin | 0.33 | 0.2 |
6′-Epi-pravastatin | 0.92 | 0.3 |
Pravastatin | 1.0 | — |
Pravastatin related compound A | 1.1 | 0.2 |
Pentanoyl impuritya | 1.2 | 0.2 |
Pravastatin lactone | 1.8 | 0.2 |
Compactin | 3.1 | 0.2 |
Any unspecified impurity | — | 0.10 |
Total impurities | — | 0.6 |
a (3R,5R)-3,5-Dihydroxy-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-[[(2S)-2-methylpentanoyl]oxy]-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]heptanoic acid.
4 SPECIFIC TESTS
4.1 Limit of Alcohol (if present)
Standard stock solution 1: Pipet 2 mL of dehydrated alcohol into a 100-mL volumetric flask, and dilute with water to volume. Standard stock solution 2: Pipet 10 mL of Standard stock solution 1 into a 100-mL volumetric flask, and dilute with water to volume. Sample stock solution: Transfer 0.2 g of Pravastatin Sodium to a 20-mL volumetric flask, and dilute with water to volume. Sample solution: Pipet 5 mL of the Sample stock solution into a vial fitted with a septum and a crimp cap, add 1 mL of water, seal the vial, and mix. Heat the sealed vial at 80° for 60 min.
Standard solution: Pipet 1 mL of Standard stock solution 2 into a vial fitted with a septum and a crimp cap, and calculate the amount of alcohol (WA ) added in grams (the specific gravity of dehydrated alcohol is 0.79 g/mL). Add 5 mL of the Sample solution to the same vial, and seal the vial. Heat the sealed vial at 80° for 60 min.
Blank solution: Pipet 6 mL of water into a vial fitted with a septum and a crimp cap, and seal the vial. Heat the sealed vial at 80° for 60 min.
4.1.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.53-mm × 30-m fused silica capillary; coated with a 3-µm lm of stationary phase G43
Temperatures
Transfer line: 85°
Injection port: 140°
Detector: 250°
Column: See Table 4.
Table 4
Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
40 | 0 | 40 | 20 |
40 | 10 | 240 | — |
240 | 0 | 240 | 20 |
Carrier gas: Helium
Flow rate: Linear velocity of 35 cm/s
Injection volume: 1 mL
Injection type: Split ratio, 1:5
4.1.2 System suitability
Sample: Blank solution
Suitability requirement: No interfering peaks are observed.
4.1.3 Analysis
Samples: Sample solution and Standard solution
Calculate the percentage of alcohol in the portion of Pravastatin Sodium taken:
Result = [rU /(rS − rU )] × (WA /W) × D × 100
rU = peak response of alcohol from the Sample solution
rS = peak response of alcohol from the Standard solution
WA = amount of alcohol added (g)
W = weight of Pravastatin Sodium taken to prepare the Sample solution (g)
D = dilution factor for the Sample solution, 4
Acceptance criteria: NMT 3.0%
4.2 Optical Rotation 〈781S〉, Procedures, Specific Rotation
Sample solution: 5 mg/mL in water
Acceptance criteria: +150° to +160° (at 20°), calculated on the anhydrous and solvent-free basis
pH 〈791〉: 7.2–9.0, in a solution (1 in 20)
Water Determination 〈921〉, Method I: NMT 4.0%
5 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers. Store as per labeling instructions. Possible storage conditions could include the following, in the presence of stability data supporting the condition: Store under nitrogen in a cold place. Store at room temperature.
Change to read:
USP Reference Standards 〈11〉
USP Pravastatin 1,1,3,3-Tetramethylbutylamine RS
2,4,4-Trimethylpentan-2-amine (3R,5R)-3,5-dihydroxy-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-{[(S)-2-methylbutanoyl]oxy}-1,2,6,7,8,8a hexahydronaphthalen-1-yl]heptanoate.
C23H36O7 · C8H19N 553.78
USP Pravastatin Sodium RS
USP Pravastatin Related Compound A RS
Sodium (3R,5R)-3,5-dihydroxy-7-[(1S,2R,3S,8S,8aR)-3-hydroxy-2-methyl-8-[[(2S)-2-methylbutanoyl]oxy]-1,2,3,7,8,8a-hexahydronaphthalen-1- yl]heptanoate.
C23H35NaO7 446.52 (ERR 1-Jun-2020)


