Pralidoxime Chloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
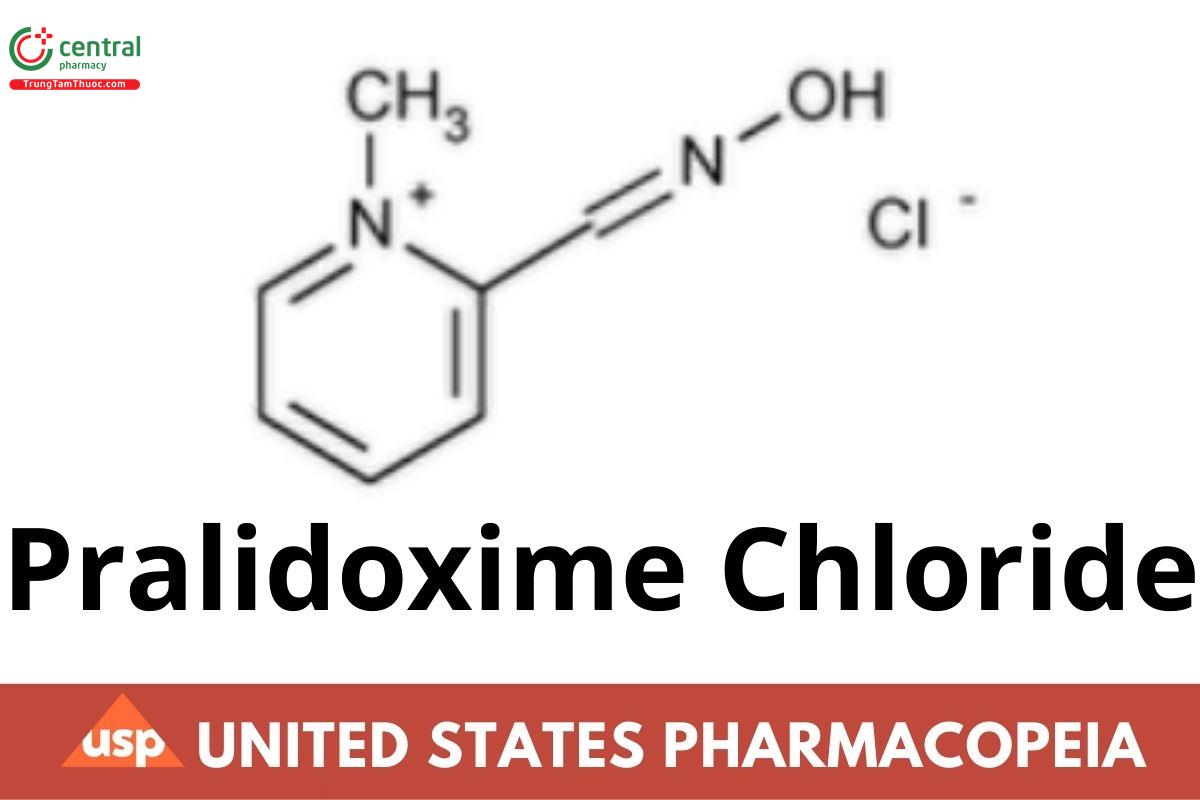
C7H9ClN2O 172.61
Pyridinium, 2-[(hydroxyimino)methyl]-1-methyl-, chloride (E)-;
(E)-2-Formyl-1-methylpyridinium chloride oxime CAS RN®: 14018-50-9.
Unspecified isomer CAS RN®: 51-15-0; UNII: 38X7XS076H.
1 DEFINITION
Pralidoxime Chloride contains NLT 97.0% and NMT 102.0% of pralidoxime chloride (C7H9ClN2O), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A, 197K, or 197M (CN 1-May-2020)
B. Identification Tests—General 〈191〉, Chemical Identification Tests, Chloride: A solution (1 in 10) meets the requirements.
C. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Solution A: Prepare a solution containing 8 mM sodium octanesulfonate and 2 mM tetraethylammonium chloride as follows. Transfer 1.87 g of sodium octanesulfonate monohydrate and 330 mg of tetraethylammonium chloride to a 1-L volumetric flask, add water to dissolve the mixture, and dilute with water to volume. Adjust with 0.01 N hydrochloric acid to a pH of 4.3.
Mobile phase: Acetonitrile and Solution A (17:83)
Diluent: Acetonitrile and water (17:83)
Standard stock solution: 1.25 mg/mL of USP Pralidoxime Chloride RS in water. Use sonication to dissolve, if necessary. Standard solution: 0.125 mg/mL of USP Pralidoxime Chloride RS in Diluent from the Standard stock solution
Sample stock solution: 1.25 mg/mL of Pralidoxime Chloride in water. Use sonication to dissolve if necessary.
Sample solution: 0.125 mg/mL of Pralidoxime Chloride in Diluent from the Sample stock solution. [Note—This solution is stable for up to 6 h when stored at room temperature protected from light.]
3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 270 nm
Column: 4.6-mm × 15-cm; 3- or 3.5-µm packing L1
Flow rate: 0.5 mL/min
Injection volume: 10 µL
3.2 System suitability
Sample: Standard solution
[Note—USP Pralidoxime Chloride RS contains pralidoxime anti-isomer as a minor component. The relative retention times for pralidoxime anti-isomer and pralidoxime are 0.9 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.0 between the pralidoxime anti-isomer and pralidoxime peaks
Tailing factor: NMT 1.5 for the pralidoxime chloride peak
Relative standard deviation: NMT 0.73% for the pralidoxime chloride peak
3.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of pralidoxime chloride (C7H9ClN2O) in the portion of Pralidoxime Chloride taken:
Result = (rU /rS ) × (CS /CU ) × 100
rU = peak response of pralidoxime from the Sample solution
rS = peak response of pralidoxime from the Standard solution
CS = concentration of USP Pralidoxime Chloride RS in the Standard solution (µg/mL)
CU = concentration of Pralidoxime Chloride in the Sample solution (µg/mL)
Acceptance criteria: 97.0%–102.0% on the dried basis
4 OTHER COMPONENTS
Content of Chloride
Sample solution: Dissolve 300 mg of Pralidoxime Chloride in 150 mL of water.
Analysis: Add 20 mL of glacial acetic acid and 10 drops of (p-tert-octylphenoxy)nonaethoxyethanol to the Sample solution, and titrate with 0.1 N silver nitrate VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary corrections. Each milliliter of 0.1 N silver nitrate is equivalent to 3.545 mg of chloride.
Acceptance criteria: 20.2%–20.8% on the dried basis
5 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.5%
Organic Impurities
Solution A, Mobile phase, Diluent, Sample solution, and Chromatographic system: Proceed as directed in the Assay. Standard stock solution: Use the Standard solution prepared as directed in the Assay.
Standard solution: 0.125 µg/mL of USP Pralidoxime Chloride RS in Diluent from the Standard stock solution Sensitivity solution: 0.0625 µg/mL of USP Pralidoxime Chloride RS in Diluent from the Standard solution
5.1 System suitability
Samples: Standard stock solution, Standard solution, and Sensitivity solution
Suitability requirements
Resolution: NLT 2.0 between the pralidoxime anti-isomer and pralidoxime peaks, Standard stock solution Relative standard deviation: NMT 5.0% for the pralidoxime chloride peak, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
5.2 Analysis
Samples: Sample solution and Standard solution
Calculate the percentage of each impurity in the portion of Pralidoxime Chloride taken:
Result = (rU /rS ) × (CS /CU ) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of pralidoxime from the Standard solution
CS = concentration of USP Pralidoxime Chloride RS in the Standard solution (µg/mL)
CU = concentration of Pralidoxime Chloride in the Sample solution (µg/mL)
Acceptance criteria: See Table 1. The reporting level for impurities is 0.05%.
Table 1
Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
Pyridine-2-aldoxime | 0.7 | 0.5 |
Pralidoxime anti-isomera | 0.9 | 2 |
Pralidoxime | 1.0 | — |
Any other individual impurity | — | 0.10 |
Total impurities | — | 3 |
a(Z)-2-[(Hydroxyimino)methyl]-1-methylpyridin-1-ium chloride.
6 SPECIFIC TESTS
Loss on Drying 〈731〉
Analysis: Dry at 105° for 3 h.
Acceptance criteria: NMT 2.0%
Sterility Tests 〈71〉: Where the label states that Pralidoxime Chloride is sterile, it meets the requirements.
Bacterial Endotoxins Test 〈85〉: Where the label states that Pralidoxime Chloride is sterile or must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.10 USP Endotoxin Units/mg of pralidoxime chloride.
7 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers.
Labeling: Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP Reference Standards 〈11〉
USP Pralidoxime Chloride RS


