Povidone-Iodine
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
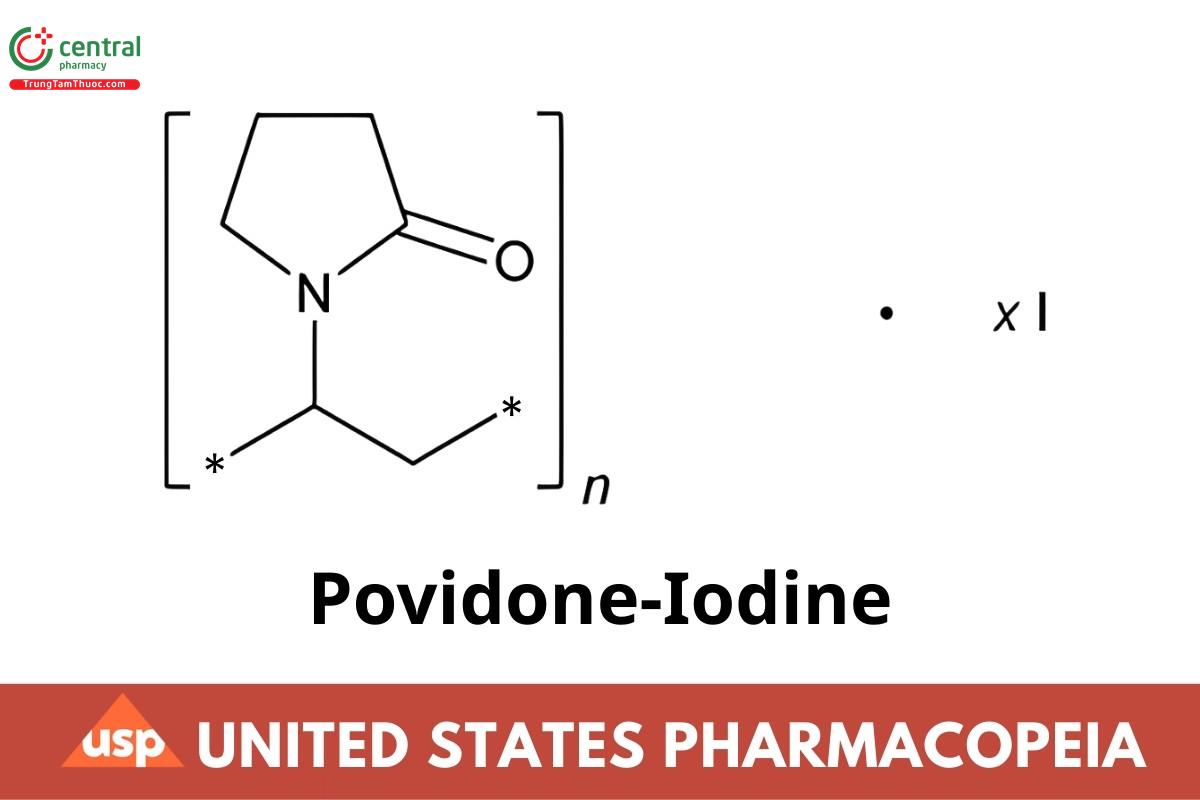
(C6H9NO)n·xI
2-Pyrrolidinone, 1-ethenyl-, homopolymer, compound with iodine;
1-Vinyl-2-pyrrolidinone polymer, compound with iodine
CAS RN®: 25655-41-8; UNII: 85H0HZU99M.
1 DEFINITION
Povidone-lodine is a complex of lodine with Povidone. It contains NLT 9.0% and NMT 12.0% of available iodine (I), calculated on the dried basis.
2 IDENTIFICATION
A.
Sample solution: 100 mg/mL
Analysis: Add 1 drop of the Sample solution to a mixture of 1 mL of starch TS and 9 mL of water.
Acceptance criteria: A deep blue color is produced.
B.
Sample solution: 100 mg/mL
Analysis: Spread 1 mL of the Sample solution over an area of 20 cm x 20 cm on a glass plate, and allow to air-dry at room temperature in an atmosphere of low humidity overnight.
Acceptance criteria: A brown, dry, non-smearing film is formed, and it dissolves readily in water.
3 ASSAY
AVAILABLE IODINE
Sample solution: Place 5 g of Povidone-lodine in a 400-mL beaker, and add 200 mL of water. Cover the beaker, and stir by mechanical means at room temperature for NMT 1 h to dissolve as completely as possible.
Titrimetric system
Mode: Direct titration
Titrant: 0.1 N sodium thiosulfate VS
Analysis: Titrate the Sample solution immediately with Titrant, adding 3 mL of starch TS as the endpoint is approached. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N sodium thiosulfate is equivalent to 12.69 mg of iodine (I).
Acceptance criteria: 9.0%-12.0% of available iodine (I) on the dried basis
4 OTHER COMPONENTS
4.1 IODIDE ION
Determination of total iodine
Sample: 500 mg of Povidone-lodine
Titrimetric system
(See Titrimetry (541), Residual Titrations.)
Mode: Residual titration
Titrant: 0.1 N silver nitrate VS
Back-titrant 0.1 N ammonium thiocyanate VS
Endpoint detection: Visual
Analysis: Dissolve the Sample in 100 mL of water in a 250-mL conical flask. Add sodium bisulfite TS until the color of iodine has disappeared. Add 25.0 mL of Titrant and 10 mL of nitric acid.
Titrate the excess silver nitrate with Back-titrant, using ferric ammonium sulfate TS as the indicator. Perform a blank determination. Each mL of 0.1 N silver nitrate is equivalent to 12.69 mg of iodine (I). From the percentage of total iodine, calculated on the dried basis, subtract the percentage of available iodine (see Assay for available iodine), to obtain the percentage of iodide ion.
Acceptance criteria: NMT 6.6% on the dried basis
4.2 NITROGEN DETERMINATION, Method II (461)
9.5%-11.5% of nitrogen (N) is found, calculated on the dried basis
5 IMPURITIES
RESIDUE ON IGNITION (281)
Sample: 2 g
Acceptance criteria: NMT 0.025%
6 SPECIFIC TESTS
LOSS ON DRYING (731)
Sample: 5.0 g
Analysis: Dry the Sample at 105° until the difference between two successive weighings at 1-h intervals is NMT 5.0 mg.
Acceptance criteria: NMT 8.0%
7 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers.


