Polyvinyl Acetate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Polyvinyl acetate is a thermoplastic polymer, represented by the formula: (C4H6O2)n in which the value of n lies between approximately 100 and 17,000.
2 IDENTIFICATION
A. PROCEDURE
Sample: 100 mg of Polyvinyl Acetate
Analysis: Dissolve the Sample in 2.5 mL of acetone, place two drops on a potassium bromide plate, and dry to evaporate the solvent.
Acceptance criteria: The IR absorption spectrum of polyvinyl acetate exhibits maxima corresponding to the same wavelengths as that of a similar preparation of USP Polyvinyl Acetate RS, treated in the same manner.
B. PROCEDURE
Sample: 0.5 g of Polyvinyl Acetate
Analysis: Saponify the Sample in a mixture of 25.0 mL of 0.5 N alcoholic potassium hydroxide and 25.0 mL of water.
Acceptance criteria: The solution so obtained meets the requirements of the tests for Identification Tests—General 〈191〉, Acetate.
3 IMPURITIES
Inorganic Impurities
RESIDUE ON IGNITION 〈281〉: NMT 0.1%
RESIDUAL PEROXIDES
Sample: 0.85 g of Polyvinyl Acetate
Analysis: Place the Sample in a borosilicate glass flask with a ground-glass neck. Add 10.0 mL of ethyl acetate, and heat under a reflux condenser with constant agitation. Allow to cool. Replace the air in the container with oxygen-free nitrogen, and add a solution of 1.0 mL of glacial acetic acid and 0.5 g of sodium iodide in 40.0 mL of water. Shake thoroughly, and allow to stand protected from light for 20 min.
Titrate with 0.005 N sodium thiosulfate VS until the yellow color is discharged. Perform a blank titration.
Acceptance criteria: The difference between the titration volumes is not greater than 1.0 mL; and NMT 100 ppm, calculated as Hydrogen peroxide, is found.
Organic Impurities
PROCEDURE: LIMIT OF VINYL ACETATE
Standard stock solution: 1.0 mg/mL of Vinyl Acetate in toluene
Standard solutions: 0.1, 0.3, 1, 3, and 10 µg/mL of vinyl acetate in toluene, prepared from Standard stock solution Sample solution: 0.1 g/mL of Polyvinyl Acetate in toluene
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Hydrogen flame ionization
Column: 0.32-mm × 30-m fused-silica capillary column, 5-µm layer of phase G1
Temperature
Detector: 250°
Injector port: 150°
Column: See the temperature program table below.
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 100 | — | 100 | 8 |
| 100 | 20 | 250 | 5 |
Carrier gas: Helium
Flow rate: Adjusted so that the vinyl acetate peak appears after about 7 min Injection size: 1.0 µL
Injection type: Split ratio is about 8:1.
System suitability
Sample: Standard solution containing 1 µg/mL of vinyl acetate in toluene
Suitability requirements
Relative standard deviation: NMT 15% Analysis
Samples: Standard solution and Sample solution
Plot the peak responses of the vinyl acetate in the Standard solutions versus the concentration, in µg/mL, of vinyl acetate, and draw the straight line best fitting the five plotted points. From the graph so obtained, determine the concentration, C, in µg/mL, of vinyl acetate in the Sample solution.
Calculate the quantity, in µg, of vinyl acetate in each g of Polyvinyl Acetate taken:
Result = (C/Cp)
C = as determined above
C = concentration of the Polyvinyl Acetate in the Sample solution (g/mL)
Acceptance criteria: NMT 5 µg/g (5 ppm) of vinyl acetate
4 SPECIFIC TESTS
4.1 FATS AND FIXED OILS, Acid Value 〈401〉
Sample: 10.0 g of Polyvinyl Acetate
Analysis: Transfer the Sample to a 250-mL glass-stoppered conical flask, dissolve in 75 mL of ethylene dichloride, add 60 mL of denatured alcoholic TS, and mix. Add 1 mL of phenolphthalein TS, and titrate with 0.02 N alcoholic potassium hydroxide VS until the solution remains faintly pink after shaking for 30 s. Perform a blank determination, and make any necessary correction.
Acceptance criteria: The acid value is NMT 0.5.
4.2 FATS AND FIXED OILS, Ester Value 〈401〉
Sample: 0.5 g of Polyvinyl Acetate
Analysis: Saponify the Sample in a mixture of 25.0 mL of 0.5 N alcoholic potassium hydroxide VS and 25.0 mL of water. Proceed as directed under Fats and Fixed Oils 〈401〉, Saponification Value, beginning with “Heat the flask on a steam bath”.
Acceptance criteria: The ester value, calculated from the Saponification Value and the Acid Value, is between 615 and 675.
4.3 LOSS ON DRYING 〈731〉
Dry 1.5 g at 100° for 2 h in a vacuum: it loses NMT 1.0% of its weight.
4.4 AVERAGE MOLECULAR WEIGHT AND MOLECULAR WEIGHT DISTRIBUTION
[CAUTION—Tetrahydrofuran (THF) is considered to be a carcinogen and embryo-fetal toxic substance. It is also a peroxide former and is flammable. A safe-handling practice must be in place in the laboratory. Carefully review appropriate Material Safety Data Sheets before use.]
Mobile phase: Tetrahydrofuran inhibited with 250 ppm butylated hydroxytoluene. Do not sparge or degas.
Standard solutions: Prepare two sets of mixtures, each set containing five narrow polystyrene standards of different known molecular weights, totaling 10 narrow polystyrene standards covering the molecular weight range from about 600 to 3,000,000 g/mol.1 Prepare each set of five narrow polystyrene standards to have a known concentration at about 0.05% (w/v) for each standard in Mobile phase.
Sample solution: Transfer 0.025 g of polyvinyl acetate to a vial, and add 10 mL of Mobile phase. Cap and mix well, using an appropriate laboratory shaker, for 1 h. Pass the polyvinyl acetate solution through a polytetrafluoroethylene filter having a porosity of 0.45 µm, discard an appropriate volume of the initial filtrate, and use the rest of the filtered solution for analysis.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.) Mode: LC
Detector: Refractive Index (RI) Detector temperature: 35°
Columns: Two 10-mm × 50-cm analytical columns; 5-µm packing L73, and a 10-mm × 10-cm, 500-Å guard column; packing L73. [NOTE—The analytical column is suitable for molecular weight ranges from 100 to 10,000,000 g/mol.]
Flow rate: 1.1 mL/min Injection size: 200 µL2
System suitability
Sample: Standard solutions
Suitability requirements
Resolution: NLT 1.7 between the polystyrene standards Analysis
Samples: Standard solutions and Sample solution
Separately inject equal volumes of the Standard solutions and the Sample solution into the chromatograph, record the chromatograms, and determine the elution peak maxima and the corresponding retention volumes for the 10 polystyrene standards.
Universal calibration: Analyze each polystyrene standard, and use a data handling system or a suitable gel permeation chromatography or size exclusion chromatography (GPC/SEC) software to compute the data and calibration. Construct the Universal calibration curve as follows, and use it in the section Data analysis for sample.
Plot log ([η] × M ) for each polystyrene standard in the Standard solutions versus its retention volume, V, in mL, at each standard peak
maximum; and construct the best cubic line fitting the 10 points. In this expression, M is the molecular weight, in g/mol, of polystyrene
standard; and [η] is the intrinsic viscosity of a polymer and is related to polymer molecular weight (M ), especially viscosity-average
molecular weight, M , by the following Mark-Houwink equation:
[η] = K × M
K = constant for a given polymer/solvent system at a specified temperature a = constant for a given polymer/solvent system at a specified temperature
For polystyrene in THF at 25°, K = 0.0128 mL/g, and a = 0.712 For polyvinyl acetate in THF at 25°, K = 0.025 mL/g, and a = 0.63
Based on the Mark-Houwink equation and the fact that Mv can represent the molecular weight (Mr), the following equation is given:
log([η] × Mr) = logK + (a + 1)log(Mr)
Mr can be obtained as Mv, a viscosity-average molecular weight of polystyrene standard.
Data analysis for sample: Analyze the polyvinyl acetate sample by identifying retention volumes Va and Vb, corresponding to the beginning and end of the polyvinyl acetate chromatogram. The baseline between Va and Vb is assumed to be linear. [NOTE—Draw a straight line between Va and Vb.] Data analysis is based on a suitable GPC/SEC computer software or a real-time data acquisition system with either offline or online data processing that is able to provide a means of determining chromatographic peak heights or integrated area segments at prescribed intervals under the SEC chromatogram and a means of handling and reporting the data. The following describes the data processes, which can be computed either by the GPC/SEC software or by an equivalent data processing system. Upon acquisition, handle the data under the polyvinyl acetate elution peak in discrete segments Ai, integrated area slices, or as digitized chromatogram heights Hi, by recording the vertical displacements between the chromatogram trace and the baseline at retention volume, Vi, over designated intervals. A minimum of 40 area segments or heights is required. Obtain the corresponding molecular weight value Mi
for Polyvinyl Acetate at its retention volume, V , from the Universal calibration curve obtained in the section Universal calibration, since the
constants K and a for Polyvinyl Acetate are known and given above.
Calculate the number-, weight-, and viscosity-average molecular weights, M , M , and M , respectively, in g/mol, of polyvinyl acetate, using
the following formula:
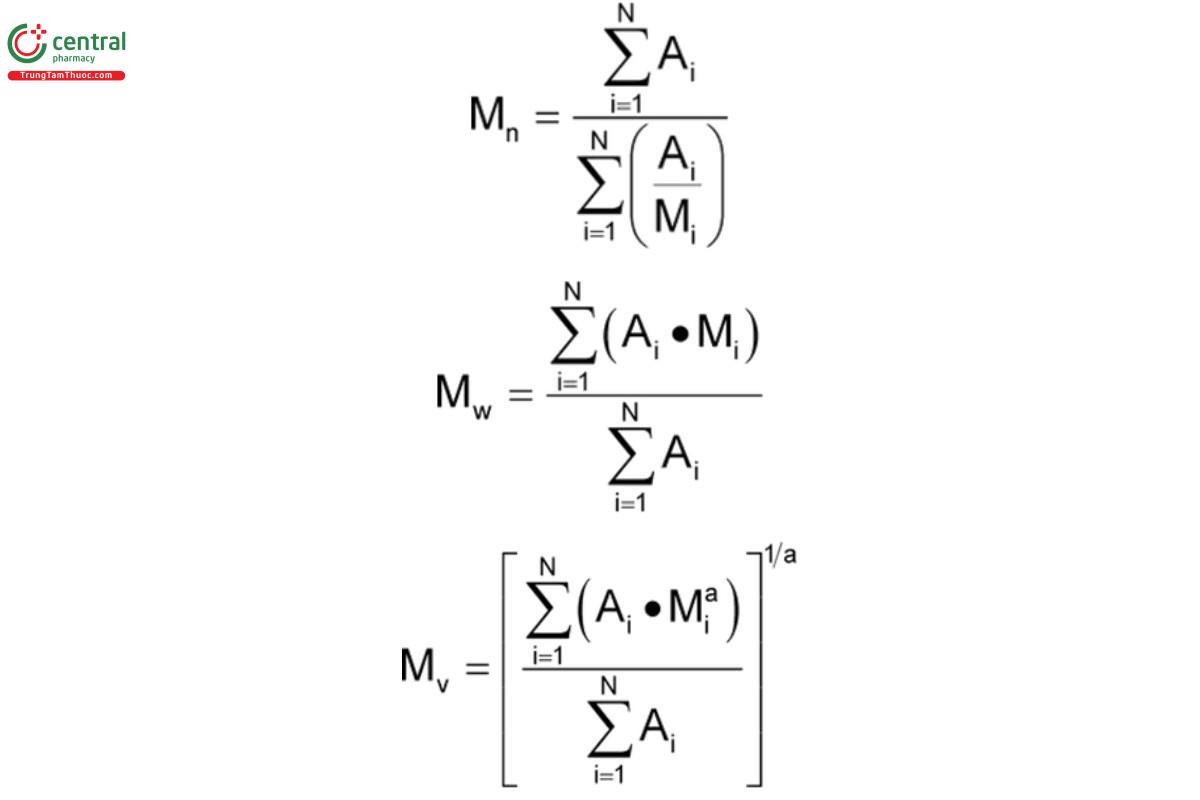
If the retention volume internal ΔV (for instance, V − V = V − V , etc.) is constant, parameters A and M are the chromatographic peak
slice area and the Polyvinyl Acetate molecular weight associated with the retention volume, V ; and N is the number of data points
obtained from the chromatogram between V and V (N ≥ 40). [NOTE—If N is sufficiently large, the use of area segments A or peak
heights H will yield equivalent results.]
Calculate the molecular weight distribution or polydispersity for Polyvinyl Acetate:
Result = M /M
Acceptance criteria: The values of weight-average molecular weight and polydispersity are, respectively, NLT 85% and NMT 115% of their respective values as stated on the label.
5 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed containers. No storage requirement specified.
LABELING: Label it to indicate its weight-average molecular weight, M , and polydispersity (M /M ).
USP REFERENCE STANDARDS 〈11〉
USP Polyvinyl Acetate RS
1 Narrow polystyrene standards are available from polymer laboratories, such as EasiCal, or are available as various individual polystyrene standards.
2 A sample loop of 400 µL and a syringe of 250 µL were used in the Analysis.


