Polymyxin B Sulfate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C56H98N16O13 1203.48
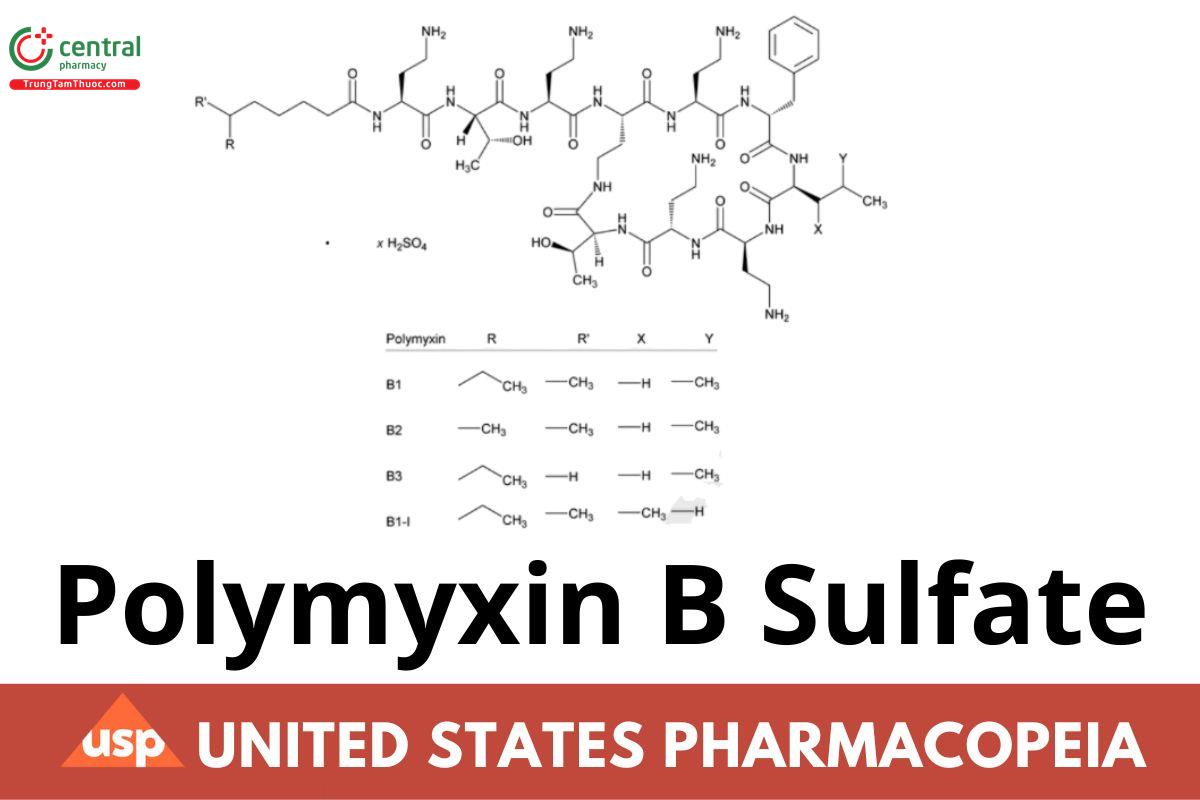
Polymyxin B1:
N-[(S)-4-Amino-1-{[(2S,3R)-1-{[(S)-4-amino-1-oxo-1-({(3S,6S,9S,12S,15S,18S,21S)-6,9,18-tris(2-aminoethyl)-15-benzyl-3-[(R)-1-hydroxyethyl]-12- isobutyl-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptaazacyclotricosan-21-yl}amino)butan-2-yl]amino}-3-hydroxy-1-oxobutan-2-yl]amino}-1- oxobutan-2-yl]-6-methyloctanamide CAS RN®: 4135-11-9.
C55H96N16O13 1189.45
Polymyxin B2:
N-[(S)-4-Amino-1-{[(2S,3R)-1-{[(S)-4-amino-1-oxo-1-({(3S,6S,9S,12S,15S,18S,21S)-6,9,18-tris(2-aminoethyl)-15-benzyl-3-[(R)-1-hydroxyethyl]-12- isobutyl-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptaazacyclotricosan-21-yl}amino)butan-2-yl]amino}-3-hydroxy-1-oxobutan-2-yl]amino}-1- oxobutan-2-yl]-6-methylheptanamide CAS RN®: 34503-87-2.
C55H96N16O13 1189.45
Polymyxin B3:
N-[(S)-4-Amino-1-{[(2S,3R)-1-{[(S)-4-amino-1-oxo-1-({(3S,6S,9S,12S,15S,18S,21S)-6,9,18-tris(2-aminoethyl)-15-benzyl-3-[(R)-1-hydroxyethyl]-12- isobutyl-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptaazacyclotricosan-21-yl}amino)butan-2-yl]amino}-3-hydroxy-1-oxobutan-2-yl]amino}-1- oxobutan-2-yl]octanamide CAS RN®: 71140-58-4.
C56H98N16O13 1203.48
Polymyxin B1-I:
N-[(S)-4-Amino-1-{[(2S,3R)-1-{[(S)-4-amino-1-oxo-1-({(3S,6S,9S,12S,15S,18S,21S)-6,9,18-tris(2-aminoethyl)-15-benzyl-3-[(R)-1-hydroxyethyl]-12- [(S)-sec-butyl]-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptaazacyclotricosan-21-yl}amino)butan-2-yl]amino}-3-hydroxy-1-oxobutan-2- yl]amino}-1-oxobutan-2-yl]-6-methyloctanamide.
Polymyxin B, sulfate;
Polymyxin B sulfate CAS RN®: 1405-20-5; UNII: 19371312D4.
Polymyxin B CAS RN®: 1404-26-8; UNII: J2VZ07J96K.
1 DEFINITION
Polymyxin B Sulfate is the sulfate salt of a kind of polymyxin, a substance produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae), or a mixture of two or more such salts. It has a potency of NLT 6000 Polymyxin B units/mg, calculated on the dried basis.
2 IDENTIFICATION
A. The retention times of the major peaks of the Sample solution correspond to those of the Standard solution, as obtained in the test for Composition of Polymyxins.
B.
Solution A: 10 mg/mL of cupric sulfate
Sample solution: Dissolve 2 mg in 5 mL of water.
Analysis: To the Sample solution add 5 mL of 2.5 N sodium hydroxide, and mix. Add 5 drops of Solution A, shaking after the addition of each drop.
Acceptance criteria: A reddish-violet color is produced.
C. Identification Tests—General 〈191〉, Sulfate: A 50-mg/mL solution of Polymyxin B Sulfate meets the requirements.
3 ASSAY
Procedure
Analysis: Proceed with Polymyxin B Sulfate as directed for Antibiotics—Microbial Assays 〈81〉.
Acceptance criteria: NLT 6000 Polymyxin B units/mg on the dried basis
4 IMPURITIES
4.1 Residue on Ignition 〈281〉
Analysis: Proceed as directed in the chapter, moistening the charred residue with 2 mL of nitric acid and 5 drops of sulfuric acid.
Acceptance criteria: NMT 5.0% where used for prescription compounding
4.2 Organic Impurities
Buffer, Mobile phase, Diluent, Standard solution, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the test for Composition of Polymyxins.
Analysis
Samples: Standard solution (the Standard solution is used to determine the disregard limit) and Sample solution
Calculate the percentage of each impurity in the portion of Polymyxin B Sulfate taken:
Result = (rU /rT ) × 100
rU = peak response of each impurity from the Sample solution
rT = sum of the responses of all peaks from the Sample solution
Acceptance criteria: Disregard any peak less than 0.007 times the peak response of the polymyxin B1 peak from the Standard solution. Any individual impurity: NMT 3.0%
Total impurities: NMT 17.0%
5 SPECIFIC TESTS
5.1 pH 〈791〉
Sample solution: 5 mg/mL
Acceptance criteria: 5.0–7.5
5.2 Loss on Drying 〈731〉
Analysis: Dry 100 mg in a capillary-stoppered bottle under vacuum at 60° for 3 h.
Acceptance criteria: NMT 7.0%
Sterility Tests 〈71〉: Meets the requirements where the label states that Polymyxin B Sulfate is sterile
5.3 Pyrogen Test 〈151〉
Sample solution: Nominally 20 × 103 Polymyxin B units/mL in pyrogen-free saline TS
Analysis: Proceed as directed in the chapter using a test dose of 1.0 mL/kg.
Acceptance criteria: Meets the requirements where intended for injectable dosage forms or where the label states that Polymyxin B Sulfate must be subjected to further processing during the preparation of injectable dosage forms
5.4 Composition of Polymyxins
Buffer: 4.5 g/L of sodium sulfate anhydrous. The pH is adjusted to 2.3 with dilute phosphoric acid (10% w/w) prior to nal dilution. Mobile phase: Acetonitrile and Buffer (20:80)
Diluent: Acetonitrile and water (20:80)
Standard solution: 0.5 mg/mL of USP Polymyxin B Sulfate RS in Diluent
Sample solution: 0.5 mg/mL of Polymyxin B Sulfate in Diluent
5.4.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 215 nm
Column: 4.6-mm × 15-cm; 3.5-µm packing L1. [Note—A 4.6-mm × 25-cm; 5-µm packing L1 column was also found to be suitable.]
Column temperature: 30°
Flow rate: 1 mL/min
Injection volume: 20 µL
Run time: 1.4 times the retention time of polymyxin B1
5.4.2 System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 3.0 between polymyxin B2 and polymyxin B3
Relative standard deviation: NMT 2.0% for the polymyxin B1 peak
5.4.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of polymyxin B1 in the portion of Polymyxin B Sulfate taken:
Result = (rU /rS ) × (CS /CU ) × P × 100
rU = peak response of polymyxin B1 from the Sample solution
rS = peak response of polymyxin B1 from the Standard solution
CS = concentration of USP Polymyxin B Sulfate RS in the Standard solution (mg/mL)
CU = concentration of Polymyxin B Sulfate in the Sample solution corrected for loss on drying (mg/mL)
P = potency of polymyxin B1 in USP Polymyxin B Sulfate RS (mg/mg)
Calculate the percentage of polymyxin B2 in the portion of Polymyxin B Sulfate taken:
Result = (rU /rS ) × (CS /CU ) × P × 100
rU = peak response of polymyxin B2 from the Sample solution
rS = peak response of polymyxin B2 from the Standard solution
CS = concentration of USP Polymyxin B Sulfate RS in the Standard solution (mg/mL)
CU = concentration of Polymyxin B Sulfate in the Sample solution corrected for loss on drying (mg/mL)
P = potency of polymyxin B2 in USP Polymyxin B Sulfate RS (mg/mg)
Calculate the percentage of polymyxin B3 in the portion of Polymyxin B Sulfate taken:
Result = (rU /rS ) × (CS /CU ) × P × 100
rU = peak response of polymyxin B3 from the Sample solution
rS = peak response of polymyxin B3 from the Standard solution
CS = concentration of USP Polymyxin B Sulfate RS in the Standard solution (mg/mL)
CU = concentration of Polymyxin B Sulfate in the Sample solution corrected for loss on drying (mg/mL)
P = potency of polymyxin B3 in USP Polymyxin B Sulfate RS (mg/mg)
Calculate the percentage of polymyxin B1-I in the portion of Polymyxin B Sulfate taken:
Result = (rU /rS ) × (CS /CU ) × P × 100
rU = peak response of polymyxin B1-I from the Sample solution
rS = peak response of polymyxin B1-I from the Standard solution
CS = concentration of USP Polymyxin B Sulfate RS in the Standard solution (mg/mL)
CU = concentration of Polymyxin B Sulfate in the Sample solution corrected for loss on drying (mg/mL)
P = potency of polymyxin B1-I in USP Polymyxin B Sulfate RS (mg/mg)
Acceptance criteria: See Table 1. Report the components on the dried basis.
Table 1
Name | Relative Retention Time | Acceptance Criteria, (%) |
Polymyxin B2a | 0.5 | — |
Polymyxin B3 | 0.6 | NMT 6.0 |
Polymyxin B1-I | 0.8 | NMT 15.0 |
Polymyxin B1a | 1.0 | — |
Sum of polymyxins B1, B2, B3, and B1-I | — | NLT 80.0 |
a These components are not reported individually. They are only reported in the sum of polymyxins.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers.
Labeling: Where packaged for prescription compounding, the label states the number of Polymyxin B units in the container and per mg, that it is not intended for manufacturing use, that it is not sterile, and that its potency cannot be assured for longer than 60 days after opening. Where it is intended for use in preparing injectable or other sterile dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable or other sterile dosage forms.
USP Reference Standards 〈11〉
USP Polymyxin B Sulfate RS


