Polyisobutylene
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
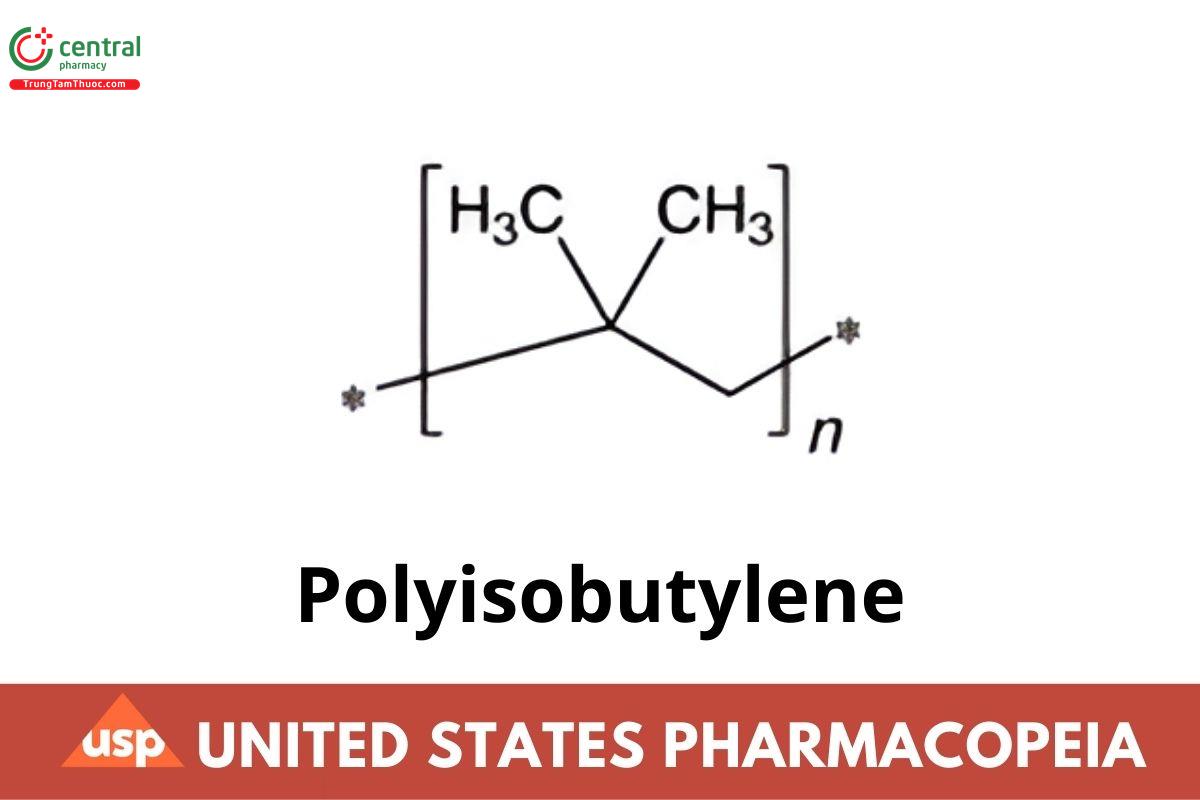
Polyisobutylene is a synthetic polymer produced by the low-temperature polymerization of isobutylene in liquid ethylene, methylene chloride, or hexane, using an aluminum-chloride or boron-trifluoride catalyst. It may contain a suitable stabilizer.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197F
Analysis: Prepare the sample by dissolving it in hot toluene and evaporating on a salt plate.
Acceptance criteria: Meets the requirements
3 IMPURITIES
Change to read:
Lead 〈251〉, Procedures, Procedure 1 (CN 1-Jun-2023)
Sample: 3.3 g
Control: 10 mL of Diluted Standard Lead Solution (10 μg of lead)
Analysis: Transfer the Sample to a porcelain dish, and heat on a hot plate until completely charred. Then heat in a mufle furnace at 480° for 8h, and cool. Cautiously add 5 mL of nitric acid, evaporate to dryness on a hot plate, then heat again in the muffle furnace for exactly 15 min, and cool. Extract the ash with two 10-mL portions of water, filtering the extract into a separator. Leach any insoluble material on the filter with 6 mL of Ammonium Citrate Solution, 2 mL of Hydroxylamine Hydrochloride Solution, and 5 mL of water, adding the filtered washings to the separator. To the resulting solution and Control continue as directed in the chapter for Procedure, beginning with “Add 2 drops of phenol red TS”.
Acceptance criteria: NMT 3 μg/g; the color generated by the Sample does not exceed that generated by the Control.
4 SPECIFIC TESTS
Viscosity - Capillary Methods 〈911〉
Solvent: Use isooctane.
Sample solution: Prepare a solution of Polyisobutylene in the Solvent having a known concentration as indicated in Table 1. The solution must be homogenous before testing. For the Polyisobutylene having a Staudinger Index of 100 and higher, add the Solvent to the weighed material, and allow it to stand in an oven at 80° for 12–24 h. [Note - A heated mechanical shaker may be used to shorten the dissolution time; it is recommended that a gentle shaker be used to avoid shearing the polymers. Take adequate precautions to prevent evaporation of the Solvent.]
Table 1
| Staudinger Indexᵃ | Concentration (g/cm³) |
|---|---|
| 25–60 | 0.01 |
| 60–100 | 0.005 |
| 100–350 | 0.002 |
| 350–700 | 0.001 |
a The Staudinger Index is equal to 100 times the intrinsic viscosity.
Analysis: Before each measurement, let the solutions be temperature equilibrated for 10 min. Using a suitable Ubbelohde capillary viscometer having dimensions such that the flow time is NLT 200 s, immersed in a controlled temperature bath, measure the flow of the
Solvent and of the Sample solution at 20°. Repeat the Analysis three times, and calculate the average.
Calculate the reduced viscosity:
J = (t/t0 − 1)/C
t = average flow time of the Sample solution (s)
t0 = average flow time of the Solvent (s)
C = concentration of the Sample solution (g/cm3)
Calculate the Staudinger Index:
J0 = J/[1 + 0.31(t/t0 − 1)]
Acceptance criteria: It is within the limits specified on the label.
Loss on Drying 〈731〉
Sample: 5 g
Analysis: Dry for 2 h at 105°.
Acceptance criteria: NMT 0.3%
5 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. No storage requirements specified.
Labeling: Label it to indicate the range for intrinsic viscosity or the range for the Staudinger Index, and the name and quantity of any added stabilizer. [Note—The Staudinger Index is equal to 100 times the intrinsic viscosity.]
USP Reference Standards 〈11〉
USP Polyisobutylene RS


