Polyethylene Glycol Monomethyl Ether
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
- DEFINITION
- ASSAY
- IMPURITIES
- RESIDUE ON IGNITION (281)
- LIMIT OF ETHYLENE GLYCOL AND DIETHYLENE GLYCOL (for Polyethylene Glycol Monomethyl Ether having a nominal molecular weight of less than 600)
- LIMIT OF ETHYLENE GLYCOL AND DIETHYLENE GLYCOL (for Polyethylene Glycol Monomethyl Ether having a nominal molecular weight of 600-1500)
- FREE ETHYLENE OXIDE AND 1,4-DIOXANE
- LIMIT OF 2-METHOXYETHANOL
- SPECIFIC TESTS
- ADDITIONAL REQUIREMENTS
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
Poly(oxy-1,2-ethanediyl), α-methyl-ω-hydroxy-;
Methoxy polyethylene glycol
CAS RN®: 9004-74-4.
1 DEFINITION
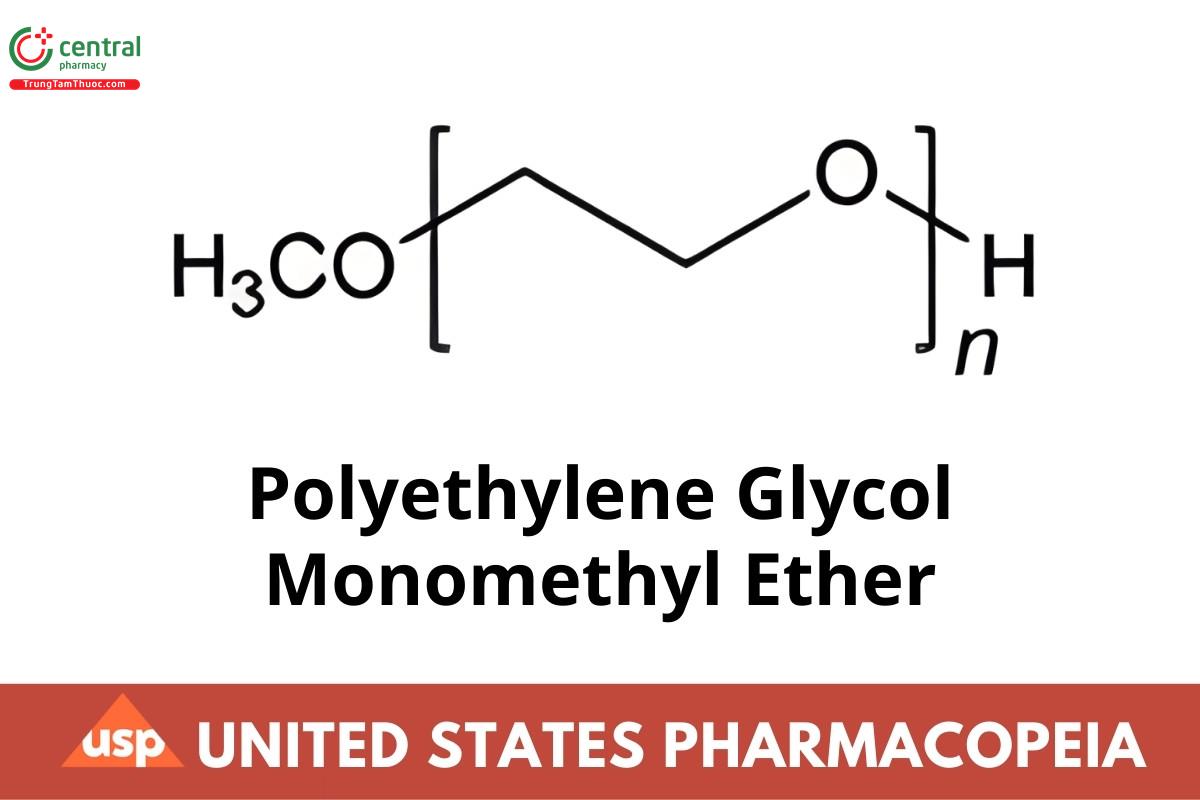
Polyethylene Glycol Monomethyl Ether is an addition polymer of ethylene oxide and methanol, represented as:
CH3(OCH2CH2)nOH
in which n represents the average number of oxyethylene groups. The average molecular weight is NLT 95.0% and NMT 105.0% of the labeled nominal value if the labeled nominal value is below 1000; it is NLT 90.0% and NMT 110.0% of the labeled nominal value if the labeled nominal value is between 1000 and 4750; and it is NLT 87.5% and NMT 112.5% of the labeled nominal value if the labeled nominal value is above 4750.
2 ASSAY
AVERAGE MOLECULAR WEIGHT
Phthalic anhydride solution: Place 49.0 g of phthalic anhydride into an amber bottle, and dissolve in 300 mL of pyridine, either from a freshly opened bottle or freshly distilled over phthalic anhydride. Shake vigorously until completely dissolved. Add 7 g of imidazole, swirl carefully to dissolve, and allow to stand for 16 h before using.
Sample solution for liquid Polyethylene Glycol Monomethyl Ethers: Carefully introduce 25.0 mL of the Phthalic anhydride solution into a dry, heat-resistant pressure bottle. Add a weighed amount of the sample, equivalent to its expected average molecular weight divided by 80. Insert the stopper in the bottle, and wrap it securely in a cloth bag.
Sample solution for solid Polyethylene Glycol Monomethyl Ethers: Carefully introduce 25.0 mL of Phthalic anhydride solution into a dry, heat-resistant pressure bottle. Add an amount of the sample, equivalent to its expected molecular weight divided by 80; however, because of limited solubility, do not use more than 25 g. Add 25 mL of pyridine, either from a freshly opened bottle or freshly distilled over phthalic anhydride, swirl to dissolve, insert the stopper in the bottle, and wrap it securely in a cloth bag.
Analysis: Immerse the bottle in a water bath maintained at 96°-100° to the same depth as that of the mixture in the bottle. Remove the bottle from the bath after 5 min, and without unwrapping, swirl for 30 s to homogenize. Heat in the water bath for 30 min (60 min for Polyethylene Glycol Monomethyl Ethers having molecular weights of 3000 or higher), then remove from the bath, and allow to cool to room temperature. Uncap the bottle carefully to release any pressure, remove from the bag, add 10 mL of water, and swirl thoroughly. Wait for 2 min, add 0.5 mL of a solution of phenolphthalein in pyridine (1 in 100). Titrate with 0.5 N sodium hydroxide VS to the first pink color that persists for 15 s, recording the volume, in mL, of 0.5 N sodium hydroxide required as VS. Perform a blank determination on 25.0 mL of Phthalic anhydride solution plus any additional pyridine added to the bottle, and record the volume, in mL, of 0.5 N sodium hydroxide required as VB. Calculate the average molecular weight:
Result = (1000 x W)/[(VB -VS) x N]
W = weight of Polyethylene Glycol Monomethyl Ether taken for the Sample solution (g)
VB = volume of 0.5 N sodium hydroxide consumed by the blank (mL)
VS = volume of 0.5 N sodium hydroxide consumed by the sample (mL)
N = normality of the sodium hydroxide solution
Acceptance criteria: See Table 1.
Table 1
| Label Claim (nominal value) | Acceptance Criteria (%) |
| <1000 | 95.0–105.0 |
| 1000–4750 | 90.0–110.0 |
| >4750 | 87.5–112.5 |
3 IMPURITIES
3.1 RESIDUE ON IGNITION (281)
Sample: 25 g of Polyethylene Glycol Monomethyl Ether, moistened with 2 mL of sulfuric acid in a platinum dish
Acceptance criteria: NMT 0.1%
3.2 LIMIT OF ETHYLENE GLYCOL AND DIETHYLENE GLYCOL (for Polyethylene Glycol Monomethyl Ether having a nominal molecular weight of less than 600)
Standard solution: 500 µg/mL of ethylene glycol and 500 µg/mL of diethylene glycol in water
Sample solution: 400 mg/mL of Polyethylene Glycol Monomethyl Ether in water
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 3-mm x 1.0-m; 60- to 80-mesh support S2
Temperatures
Column: 200°
Injection port: 260°
Carrier gas: Nitrogen or another suitable inert gas
Flow rate: 20 mL/min
Injection volume: 1.0 µL
Analysis
Samples: Standard solution and Sample solution
[NOTE-The elution order is ethylene glycol, diethylene glycol, and polyethylene glycol monomethyl ether.]
Calculate the percentage of ethylene glycol and diethylene glycol in the portion of Polyethylene Glycol Monomethyl Ether taken:
Result = (rU/rS) x (CS/CU) x 100
rU = peak height of ethylene glycol or diethylene glycol from the Sample solution
rS = peak height of ethylene glycol or diethylene glycol from the Standard solution
CS = concentration of ethylene glycol or diethylene glycol in the Standard solution
CU = concentration of Polyethylene Glycol Monomethyl Ether in the Sample solution
Acceptance criteria: NMT 0.25% of combined ethylene glycol and diethylene glycol
3.3 LIMIT OF ETHYLENE GLYCOL AND DIETHYLENE GLYCOL (for Polyethylene Glycol Monomethyl Ether having a nominal molecular weight of 600-1500)
Solution A: 62.5 mg/mL of ceric ammonium nitrate in 0.25 N nitric acid. Use within 3 days.
Solution B: Freshly distilled acetonitrile and water (50:50)
Standard solution: 2.5 mg/mL of diethylene glycol in Solution B
Sample solution: Dissolve 50.0 g of Polyethylene Glycol Monomethyl Ether in 75 mL of diphenyl ether, previously warmed if necessary, to melt the crystals, in a 250-mL distilling flask. Slowly distill at a pressure of 1-2 mm of mercury into a receiver graduated to 100 mL in 1-mL subdivisions until 25 mL of distillate has been collected. Add 20.0 mL of water to the distillate, shake vigorously, and allow the layers to separate. Cool in an ice bath to solidify the diphenyl ether and facilitate its removal. Filter the separated aqueous layer, wash the diphenyl ether with 5.0 mL of ice-cold water, pass the washings through the filter, and collect the filtrate and washings in a 25-mL volumetric flask. Warm to room temperature, and dilute with water to volume, if necessary. Mix this solution with 25.0 mL of freshly distilled acetonitrile in a glass-stoppered, 125-mL conical flask.
Instrumental conditions
Mode: Vis
Analytical wavelength: About 450 nm
Cell: 1 cm
Blank: Solution A and Solution B (60:40)
Analysis
Samples: Standard solution, Sample solution, and Blank
Transfer 10.0 mL each of the Standard solution and the Sample solution to separate 50-mL flasks, each containing 15.0 mL of Solution A. Within 2-5 min, determine the absorbances of the Samples.
Acceptance criteria: The absorbance of the Sample solution does not exceed that of the Standard solution, corresponding to NMT 0.25% of combined ethylene glycol and diethylene glycol.
3.4 FREE ETHYLENE OXIDE AND 1,4-DIOXANE
Stripped MPEG 350: Into a 5000-mL 4-neck, round-bottom flask, equipped with a stirrer, a thermometer, a gas dispersion tube, a dry ice trap, a vacuum outlet, and a heating mantle, place 3000 g of Polyethylene Glycol Monomethyl Ether 350. At room temperature, evacuate the flask carefully to a pressure of less than 1 mm of mercury, applying the vacuum slowly while observing for excessive foaming due to entrapped gases. After any foaming has subsided, sparge with nitrogen, allowing the pressure to rise to 10 mm of mercury. Heat the flask to 130° while increasing the pressure to 60 mm of mercury. Continue stripping for 4 h, then cool to room temperature. Shut off the vacuum pump, and bring the flask pressure back to atmospheric pressure while maintaining nitrogen sparging. Remove the sparging tube with the gas still flowing, then turn off the gas flow. Transfer the Stripped MPEG 350 to a suitable nitrogen-filled container.
Standard solutions: [CAUTION-Ethylene oxide and 1,4-dioxane are toxic and flammable. Prepare these solutions in a well-ventilated fume hood.] To a known weight of Stripped MPEG 350 in a vial that can be sealed add a suitable quantity of 1,4-dioxane. Determine the amount added by weight difference. Using the special handling described in the following, complete the preparation. Ethylene oxide is a gas at room temperature. It is usually stored in a lecture-type gas cylinder or small metal pressure bomb. Chill the cylinder in a refrigerator before use. Transfer 5 mL of the liquid ethylene oxide to a 100-mL beaker chilled in wet ice. Using a gas-tight gas chromatographic syringe that has been chilled in a refrigerator, transfer a suitable amount of the liquid ethylene oxide into the mixture. Immediately seal the vial, and shake. Determine the amount added by weight difference. By appropriate dilution with Stripped MPEG 350, prepare four solutions, covering a range of 5-20 ppm for the two components added to the matrix (e.g., 5, 10, 15, and 20 ppm). Transfer 1.0 mL of each of these solutions to separate 22-mL pressure headspace vials. Seal each with a silicone septum, star spring, and pressure relief safety aluminum sealing cap. Crimp the cap closed with a cap-sealing tool.
Sample solution: Transfer 1 ± 0.01 g of Polyethylene Glycol Monomethyl Ether to a 22-mL pressure headspace vial. Seal, cap, and crimp as directed for the Standard solutions.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: GC (equipped with a balanced pressure automatic headspace sampler)
Detector: Flame ionization
Column: 50-m x 0.32-mm fused silica; bonded phase G27 in a 5-µm film thickness
Temperatures
Detector: 250°
Transfer line: 140°
Column: See Table 2.
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 70 | 10 | 250 | — |
Carrier gas: Helium
Flow rate: 0.8 mL/min
Calibration: Place the vials containing the Standard solutions in the automated sampler, and start the sequence so that each vial is heated at 110° for 30 min before a suitable portion of its headspace is injected into the chromatograph. Set the automatic sampler for a needle withdrawal time of 0.3 min, a pressurization time of 1 min, an injection time of 0.08 min, and a vial pressure of 22 psig with the vial vent off. Obtain the peak areas for ethylene oxide and 1,4-dioxane, which have relative retention times of 1.0 and 3.1, respectively. Plot the area versus parts per million on linear graph paper, and draw the best straight line through the points. On the two Calibration plots, no point digresses from its line by more than 10%.
Analysis: Place the vial containing the Sample solution in the automatic sampler, and chromatograph its headspace as done for the Standard solutions. Obtain the peak areas of each of the components, and read the concentrations directly from the Calibration plots.
Acceptance criteria: NMT 10 ppm of ethylene oxide or 1,4-dioxane
3.5 LIMIT OF 2-METHOXYETHANOL
Stripped MPEG 350 and Sample solution: Prepare as directed in the test for Free Ethylene Oxide and 1,4-Dioxane.
Standard solutions: [CAUTION-2-Methoxyethanol is toxic and flammable. Prepare these solutions in a well-ventilated fume hood.] To a known weight of Stripped MPEG 350 in a vial that can be sealed add a suitable quantity of 2-methoxyethanol. Determine the amount added by weight difference. By appropriate dilution with Stripped MPEG 350, prepare four solutions, covering a range of 5-20 ppm (e.g., 5, 10, 15, and 20 ppm). Transfer 1.0 mL of each of these solutions to separate 22-mL pressure headspace vials. Seal each with a silicone septum, star spring, and pressure relief safety aluminum sealing cap. Crimp the cap closed with a cap-sealing tool.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: GC (equipped with a balanced pressure automatic headspace sampler)
Detector: Flame ionization
Column: 15-m x 0.53-mm fused silica capillary; bonded phase G16 in a 1-µm film thickness
Temperatures
Detector: 275°
Transfer line: 140°
Column: See Table 3.
Table 3
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 50 | — | 50 | 2 |
| 70 | 10 | 250 | — |
Carrier gas: Helium
Flow rate: 15 mL/min
Calibration: Place the vials containing the Standard solutions in the automated sampler, and start the sequence so that each vial is heated at 100° for 20 min before a suitable portion of its headspace is injected into the chromatograph. Set the automatic sampler for a needle withdrawal time of 0.3 min, a pressurization time of 1 min, an injection time of 0.08 min, and a vial pressure of 22 psig with the vial vent off. Obtain the peak area for 2-methoxyethanol. Plot the area versus ppm on linear graph paper, and draw the best straight line through the points. On the Calibration plot, no point digresses from its line by more than 10%.
Analysis: Place the vial containing the Sample solution in the automatic sampler, and chromatograph its headspace as done for the Standard solutions. Obtain the peak area, and read the concentration directly from the Calibration plot.
Acceptance criteria: NMT 10 ppm
4 SPECIFIC TESTS
4.1 PH (791)
Sample solution: 5.0 g of Polyethylene Glycol Monomethyl Ether in 100 mL of carbon dioxide-free water. Add 0.30 mL of saturated potassium chloride solution.
Acceptance criteria: 4.5-7.5
4.2 COMPLETENESS AND COLOR OF SOLUTION
Sample solution: 5 g of Polyethylene Glycol Monomethyl Ether in 50 mL of water
Acceptance criteria: The resulting solution is colorless, and is clear for liquid grades and NMT slightly hazy for solid grades.
4.3 VISCOSITY-CAPILLARY METHODS (911)
Determine its viscosity, using a capillary viscometer giving a flow time of NLT 200 s and a liquid bath maintained at 98.9 ± 0.3°. The viscosity is within the limits specified in Table 4. For a Polyethylene Glycol Monomethyl Ether not listed in Table 4, calculate the limits by interpolation.
Table 4
| Nominal Average Molecular Weight | Viscosity Range (centistokes) | Nominal Average Molecular Weight | Viscosity Range (centistokes) |
| 350 | 3.5–4.5 | 2750 | 50–78 |
| 450 | 4.9–6.0 | 3000 | 60–95 |
| 550 | 6.1–7.3 | 3250 | 72–113 |
| 650 | 7.9–9.2 | 3500 | 85–133 |
| 750 | 9.7–11.1 | 3750 | 99–155 |
| 850 | 11.5–13.1 | 4000 | 114–178 |
| 950 | 13.3–15.2 | 4250 | 130–204 |
| 1000 | 13.3–17.3 | 4500 | 148–231 |
| 1100 | 15.0–19.7 | 4750 | 167–260 |
| 1200 | 16.9–22.1 | 5000 | 175–305 |
| 1300 | 18.8–24.6 | 5500 | 215–375 |
| 1400 | 20.7–27.1 | 6000 | 260–455 |
| 1500 | 23–30 | 6500 | 310–545 |
| 1600 | 25–33 | 7000 | 365–640 |
| 1700 | 27-35 | 7500 | 425–745 |
| 1800 | 29-38 | 8000 | 490–860 |
| 1900 | 31-41 | 8500 | 560–980 |
| 2000 | 33-44 | 9000 | 640–1110 |
| 2250 | 36–54 | 9500 | 715–1250 |
| 2500 | 40–64 | 10000 | 775–1475 |
5 ADDITIONAL REQUIREMENTS
5.1 PACKAGING AND STORAGE
Preserve in tight containers.
5.2 LABELING
Label it to state, as part of the official title, the average nominal molecular weight of the Polyethylene Glycol Monomethyl Ether.


