Plerixafor
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
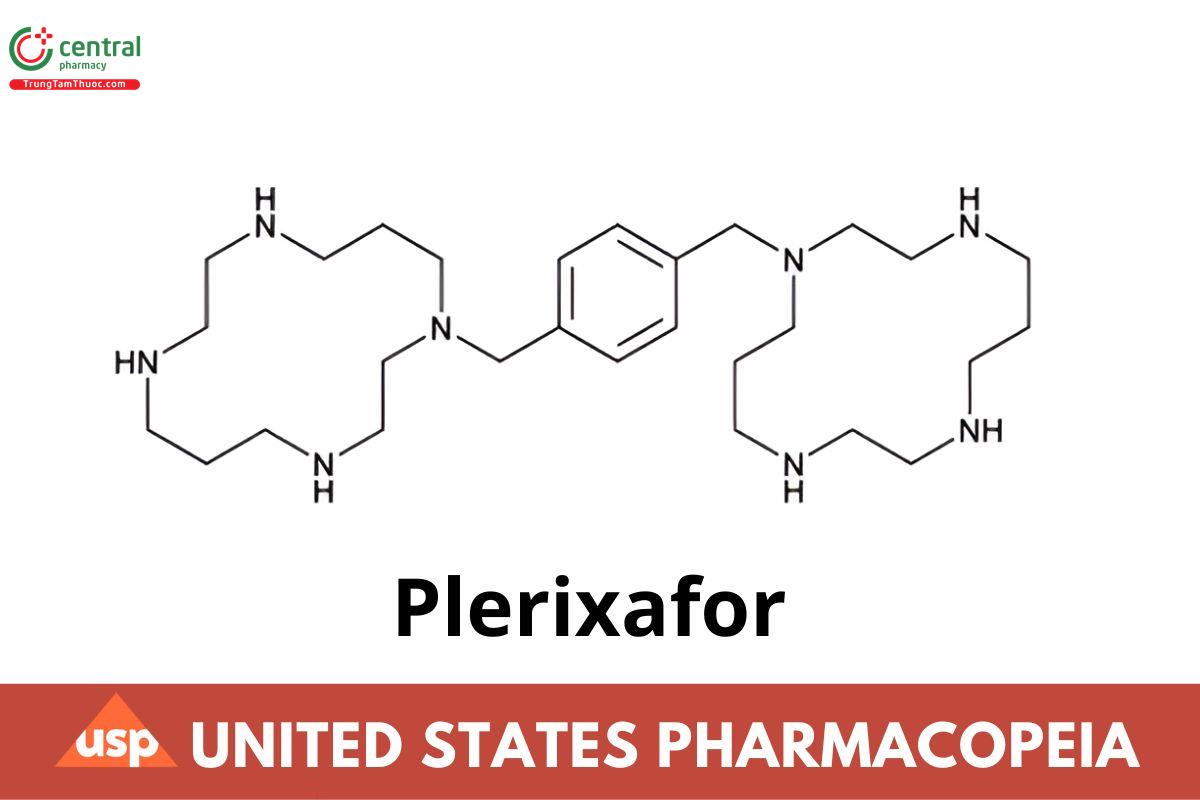
C28H54N8 502.80
1,4,8,11-Tetraazacyclotetradecane, 1,1'-[1,4-phenylenebis(methylene)]bis-;
1,4-Bis[(1,4,8,11-tetraazacyclotetradecan-1-yl)methyl]benzene CAS RN®: 110078-46-1; UNII: S915P5499N.
1 DEFINITION
Plerixafor contains NLT 97.0% and NMT 103.0% of plerixafor (C28H54N8), calculated on the anhydrous basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A or 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Solution A: 0.1% (v/v) trifluoroacetic acid in water
Solution B: Methanol and water (50:50). To each liter of the solution, add 1 mL of trifluoroacetic acid.
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) | Flow Rate (mL/min) |
|---|---|---|---|
| 0 | 80 | 20 | 0.8 |
| 15 | 80 | 20 | 0.8 |
| 41 | 47 | 53 | 0.8 |
| 43 | 47 | 53 | 0.8 |
| 44 | 10 | 90 | 0.8 |
| 45 | 10 | 90 | 1.0 |
| 46 | 80 | 20 | 1.0 |
| 50 | 80 | 20 | 0.8 |
Diluent: 0.05 N hydrochloric acid
Standard solution: 1 mg/mL of USP Plerixafor RS in Diluent. Sonicate to dissolve.
Sample solution: 1 mg/mL of Plerixafor in Diluent. Sonicate to dissolve.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 230 nm
Columns
Guard: 4.6-mm × 12.5-mm; 5-μm packing L7
Analytical: 4.6-mm × 15-cm; 3.5-μm packing L7
Column temperature: 40°
Flow rate: See Table 1.
Injection volume: 20 μL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 5.0
Relative standard deviation: NMT 1.10%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of plerixafor (C28H54N8) in the portion of Plerixafor taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of plerixafor from the Sample solution
rS = peak response of plerixafor from the Standard solution
CS = concentration of USP Plerixafor RS in the Standard solution (mg/mL)
CU = concentration of Plerixafor in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–103.0% on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Organic Impurities
Solution A, Solution B, Mobile phase, Diluent, Standard solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
System suitability solution: 1 mg/mL of USP Plerixafor System Suitability Mixture RS in Diluent. Sonicate to dissolve.
Sensitivity solution: 0.0005 mg/mL of USP Plerixafor RS in Diluent from the Standard solution
System suitability
[Note - Column conditioning may be performed as needed to meet the system suitability requirements using 1.6 mg/mL of USP Plerixafor System Suitability Mixture RS in water as a column conditioning solution. Flush the column with 0.1% v/v trifluoroacetic acid in methanol for 1 h. Equilibrate the column with the starting Mobile phase gradient for 30 min. Carry out multiple injections of the column conditioning solution until the resolution criterion of NLT 1.0 between plerixafor 4-benzyl analog and plerixafor 8-benzyl analog is met.]
Samples: Standard solution, System suitability solution, and Sensitivity solution
[Note - The relative retention times in Table 2 are provided as information that could aid in peak assignment.]
Table 2
| Name | Relative Retention Time |
|---|---|
| Plerixafor benzyl alcoholᵃ | 0.85 |
| Plerixafor | 1.00 |
| Plerixafor 4-benzyl analogᵇ | 2.54 |
| Plerixafor 8-benzyl analogᶜ | 2.58 |
| Plerixafor 11-benzyl analogᵈ | 2.90 |
a{4-[(1,4,8,11-Tetraazacyclotetradecan-1-yl)methyl]phenyl}methanol.
b 1,4-Bis{4-[(1,4,8,11-tetraazacyclotetradecan-1-yl)methyl]benzyl}-1,4,8,11-tetraazacyclotetradecane.
c 1,8-Bis{4-[(1,4,8,11-tetraazacyclotetradecan-1-yl)methyl]benzyl}-1,4,8,11-tetraazacyclotetradecane.
d 1,11-Bis{4-[(1,4,8,11-tetraazacyclotetradecan-1-yl)methyl]benzyl}-1,4,8,11-tetraazacyclotetradecane.
Suitability requirements
Resolution: NLT 1.0 between plerixafor 4-benzyl analog and plerixafor 8-benzyl analog; NLT 1.0 between plerixafor and plerixafor benzyl alcohol, System suitability solution
Relative standard deviation: NMT 1.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each specified and unspecified impurity in the portion of Plerixafor taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU = peak response of each specified or unspecified impurity from the Sample solution
rS = peak response of plerixafor from the Standard solution
CS = concentration of USP Plerixafor RS in the Standard solution (mg/mL)
CU = concentration of Plerixafor in the Sample solution (mg/mL)
F = relative response factor (see Table 3)
Acceptance criteria: See Table 3. The reporting threshold is 0.05%.
Table 3
| Name | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|
| Plerixafor benzyl alcohol | 0.77 | 0.15 |
| Plerixafor 4-benzyl analog | 1.0 | 0.26 |
| Plerixafor 8-benzyl analog | 1.2 | 0.34 |
| Plerixafor 11-benzyl analog | 1.1 | 0.12 |
| Any unspecified impurity | 1.0 | 0.10 |
| Total impuritiesᵃ | – | 1.2 |
a Sum of the results obtained in the Organic Impurities and Limit of Cyclam tests.
Limit of Cyclam
Solution A: 0.1% (v/v) trifluoroacetic acid in water
Solution B: 0.1% (v/v) trifluoroacetic acid in acetonitrile
Mobile phase: See Table 4.
Table 4
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 95 | 5 |
| 15 | 60 | 40 |
| 20 | 95 | 5 |
| 30 | 95 | 5 |
Derivatization solution: 20 mg/mL of cupric acetate monohydrate in water
Standard solution: 0.005 mg/mL of USP Cyclam RS in water
Sample solution: 1 mg/mL of Plerixafor in water
Derivatized standard solution: 0.0025 mg/mL of cyclam prepared as follows. Mix 2 mL of the Standard solution with 2 mL of the Derivatization solution in a suitable head space vial. Crimp to seal the vial and place the vial in a 55° water bath for 15 min. Cool the vial and use for analysis.
Derivatized sample solution: 0.5 mg/mL of plerixafor prepared as follows. Mix 2 mL of the Sample solution with 2 mL of the Derivatization solution in a suitable head space vial. Crimp to seal the vial and place the vial in a 55° water bath for 15 min. Cool the vial and use for analysis.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 266 nm
Column: 4.6-mm × 15-cm; 5-μm packing L1
Flow rate: 1 mL/min
Injection volume: 20 μL
System suitability
Sample: Derivatized standard solution
Suitability requirements
Relative standard deviation: NMT 10.0%
Analysis
Samples: Derivatized standard solution and Derivatized sample solution
Calculate the percentage of cyclam in the portion of Plerixafor taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of cyclam from the Derivatized sample solution
rS = peak response of cyclam from the Derivatized standard solution
CS = concentration of USP Cyclam RS in the Derivatized standard solution (mg/mL)
CU = concentration of Plerixafor in the Derivatized sample solution (mg/mL)
Acceptance criteria: NMT 0.10%
5 SPECIFIC TESTS
Water Determination 〈921〉, Method I, Method Ic: NMT 1.5%
Bacterial Endotoxins Test 〈85〉: Where the label states that Plerixafor is sterile or must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement under the relevant dosage form monograph(s) in which Plerixafor is used can be met.
Microbial Enumeration Tests 〈61〉 and Tests for Specified Microorganisms 〈62〉: The total aerobic microbial count does not exceed 102 cfu/g. The total yeasts and molds count does not exceed 102 cfu/g.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers at controlled room temperature.
Labeling: Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP Reference Standards 〈11〉
USP Cyclam RS
1,4,8,11-Tetraazacyclotetradecane.
C10H24N4 200.33
USP Plerixafor RS
USP Plerixafor System Suitability Mixture RS
Contains a mixture of the following 5 compounds:
Plerixafor.
Plerixafor 4-benzyl analog: 1,4-Bis{4-[(1,4,8,11-tetraazacyclotetradecan-1-yl)methyl]benzyl}-1,4,8,11-tetraazacyclotetradecane.
Plerixafor 8-benzyl analog: 1,8-Bis{4-[(1,4,8,11-tetraazacyclotetradecan-1-yl)methyl]benzyl}-1,4,8,11-tetraazacyclotetradecane.
Plerixafor 11-benzyl analog: 1,11-Bis{4-[(1,4,8,11-tetraazacyclotetradecan-1-yl)methyl]benzyl}-1,4,8,11-tetraazacyclotetradecane.
Plerixafor benzyl alcohol: {4-[(1,4,8,11-Tetraazacyclotetradecan-1-yl)methyl]phenyl}methanol.


