Pentamidine Isethionate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
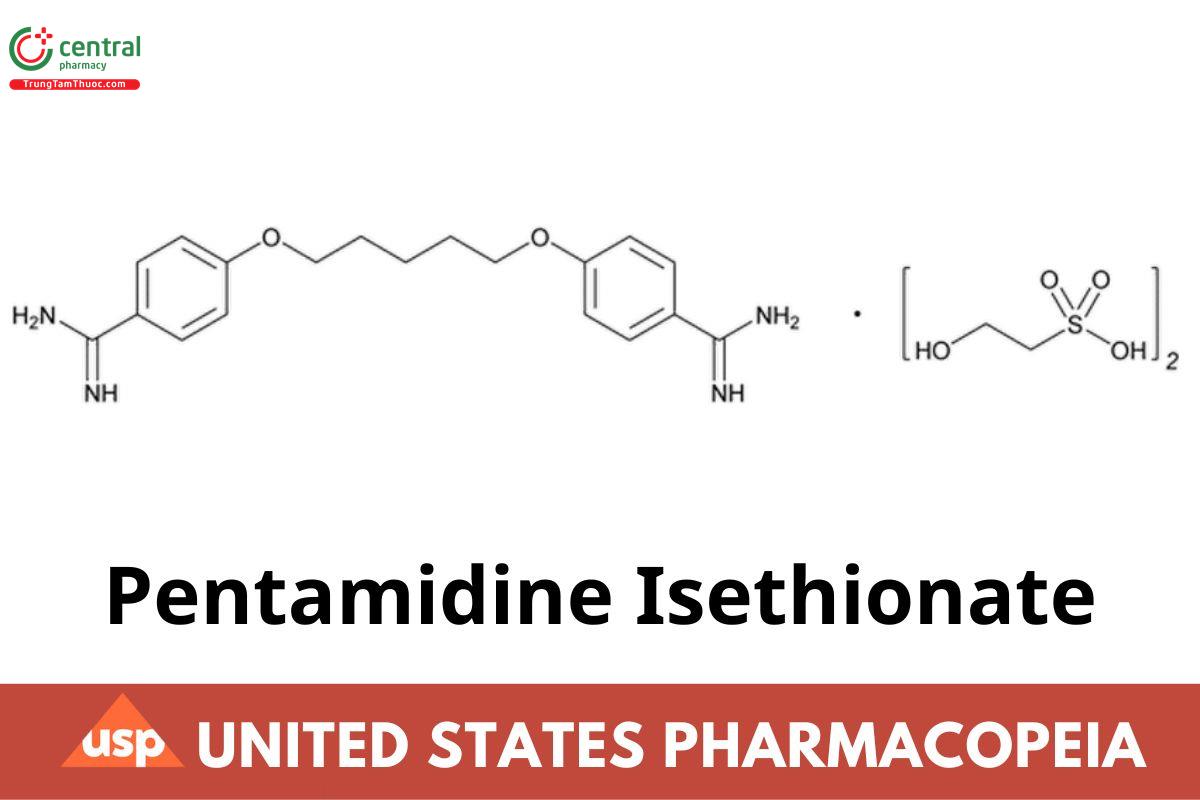
C19H24N4O2 · (C2H6O4S)2 592.68
Ethanesulfonic acid, 2-hydroxy-, compd. with 4,4'-[1,5-pentanediylbis(oxy)]bis [benzenecarboximidamide]; 4,4'-(Pentane-1,5-diylbis(oxy))dibenzimidamide bis(2-hydroxyethanesulfonate) CAS RN®: 140-64-7; UNII: V2P3K60DA2.
1 DEFINITION
Pentamidine Isethionate contains NLT 98.5% and NMT 101.5% of C19H24N4O2 · (C2H6O4S)2, calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B. Oxygen-Flask Combustion 〈471〉
Barium chloride solution: 60 mg/mL of barium chloride in water
Analysis: Burn 150 mg, using 10 mL of 3% Hydrogen peroxide as the absorbing liquid. When the process is complete, acidify with 1 mL of diluted hydrochloric acid, and add 1 mL of the Barium chloride solution.
Acceptance criteria: A white precipitate is formed.
C. The retention time of the pentamidine isethionate peak of the Sample solution corresponds to that of the Standard solution, as obtained in the test for Organic Impurities.
3 ASSAY
Procedure
Sample solution: 5 mg/mL in dimethylformamide. Add 0.25 mL of thymol blue TS.
Analysis: Titrate under a stream of nitrogen with 0.1 M tetrabutylammonium hydroxide VS, determining the endpoint until the color changes to intense blue. Perform a blank determination, and make any necessary correction (see Titrimetry 〈541〉). Each mL of 0.1 M tetrabutylammonium hydroxide is equivalent to 29.63 mg of C19H24N4O2 · (C2H6O4S)2.
Acceptance criteria: 98.5%–101.5% on the dried basis
4 IMPURITIES
Inorganic Impurities
Residue on Ignition 〈281〉
Acceptance criteria: NMT 0.1% on a 1-g sample
Organic Impurities
Procedure
Buffer: 30 mg/mL of ammonium acetate in water, adjusted with triethylamine to a pH of 7.5
Mobile phase: Methanol and Buffer (65:35)
System suitability solution: Prepare 40.0 mL of a 2.5 mg/mL solution of USP Pentamidine Isethionate RS in water. Adjust with 0.2 M sodium hydroxide to a pH of 10.5, and boil under reflux for 20 min. Cool, and dilute with water to 50.0 mL. Transfer quantitatively 1 mL of this solution to a 50-mL volumetric ask, and dilute with Mobile phase to volume.
Standard solution: 2 µg/mL of USP Pentamidine Isethionate RS in Mobile phase
Sample solution: 1.0 mg/mL of Pentamidine Isethionate in Mobile phase. [Note—It must be demonstrated that the nal product does not contain a detectable amount of alkyl 2-hydroxyethanesulphonates, a potential in-process impurity.]
4.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 265 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Flow rate: 1 mL/min
Injection size: 10 µL
Run time: 3.5 times the retention time of pentamidine
4.2 System suitability
Sample: System suitability solution
Suitability requirements
Resolution: NLT 2 between the two major peaks.
[Note—The chromatogram shows two major peaks.]
4.3 Analysis
Samples: Standard solution and Sample solution
Acceptance criteria
Individual impurities: NMT 0.4%. [Note—Exclude any other peak producing a response of less than 0.02%.] Total impurities: NMT 0.7%
5 SPECIFIC TESTS
pH 〈791〉: 4.5–6.5, in a carbon dioxide-free aqueous solution containing 50 mg/mL of Pentamidine Isethionate
Loss on Drying 〈731〉: Dry at 105°: it loses NMT 4.0% of its weight.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers, protected from light. Store at controlled room temperature. • USP Reference Standards 〈11〉
USP Pentamidine Isethionate RS
C19H24N4O2 · (C2H6O4S)2


