Penicillin V
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
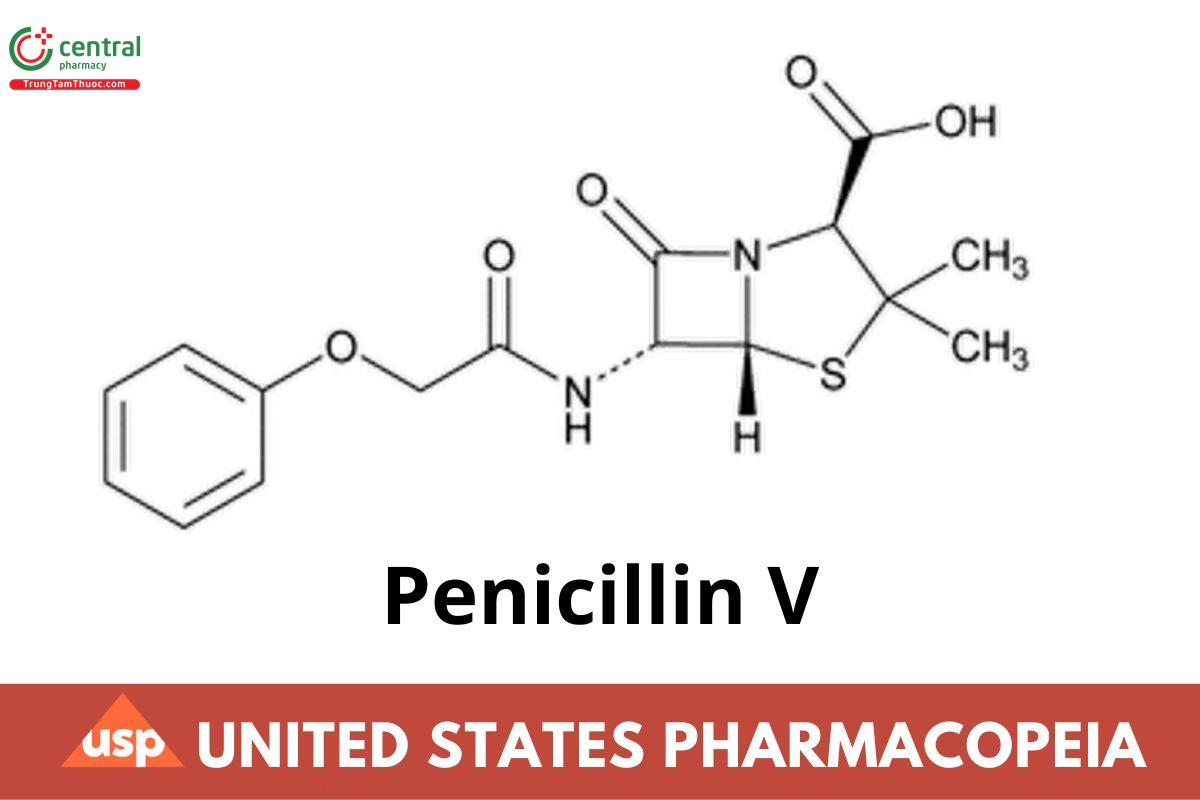
C16H18N2O5S 350.39
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-7-oxo-6-[(phenoxyacetyl)amino]-, [2S-(2α,5α,6β)]-; (2S,5R,6R)-3,3-Dimethyl-7-oxo-6-(2-phenoxyacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid CAS RN®: 87-08-1; UNII: Z61I075U2W.
1 DEFINITION
Penicillin V has a potency of NLT 1525 and NMT 1780 Penicillin V Units/mg.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
Sample: Do not dry.
Acceptance criteria: Meets the requirements
3 ASSAY
Procedure
Mobile phase: Acetonitrile, glacial acetic acid, and water (350:5.75:650)
System suitability solution: 2.5 mg/mL each of USP Penicillin G Potassium RS and USP Penicillin V Potassium RS in Mobile phase Standard solution: 2.5 mg/mL of USP Penicillin V Potassium RS in Mobile phase
Sample solution: 2.5 mg/mL of Penicillin V in Mobile phase
3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4-mm × 30-cm; packing L1
Flow rate: 1 mL/min
Injection volume: 10 µL
3.2 System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for p-hydroxypenicillin V, penicillin G, and penicillin V are about 0.4, 0.8, and 1.0, respectively.]
Suitability requirements
Resolution: NLT 3.0 between penicillin G and penicillin V, System suitability solution
Column efficiency: NLT 1800 theoretical plates, System suitability solution
Relative standard deviation: NMT 1.0% for penicillin V potassium, Standard solution
3.3 Analysis
Samples: Standard solution and Sample solution
Calculate the potency of penicillin V potassium, in Penicillin V Units/mg, in the portion of Penicillin V taken:
Result = (rU /rS) × (CS /CU ) × P
rU = sum of the peak responses of p-hydroxypenicillin V and penicillin V from the Sample solution
rS = sum of the peak responses of p-hydroxypenicillin V and penicillin V from the Standard solution
CS = concentration of USP Penicillin V Potassium RS in the Standard solution (mg/mL)
CU = concentration of Penicillin V in the Sample solution (mg/mL)
P = potency of penicillin V in USP Penicillin V Potassium RS (Penicillin V Units/mg)
Acceptance criteria: 1525–1780 Penicillin V Units/mg
4 IMPURITIES
4.1 Limit of Phenoxyacetic Acid
Mobile phase: Acetonitrile, glacial acetic acid, and water (35:1:65)
Diluent: pH 6.6 phosphate buffer (see Reagents, Indicators, and Solutions—Buffer Solutions) Standard solution: 0.1 mg/mL of phenoxyacetic acid in Diluent
Sample solution: 20.0 mg/mL of Penicillin V in Diluent. Use this solution on the day prepared.
4.1.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Flow rate: 1 mL/min
Injection volume: 20 µL
4.1.2 System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 1.5
Relative standard deviation: NMT 2.0%
4.1.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of phenoxyacetic acid in the portion of Penicillin V taken:
Result = (rU /rS) × (CS /CU ) × 100
rU = peak area of phenoxyacetic acid from the Sample solution
rS = peak area of phenoxyacetic acid from the Standard solution
CS = concentration of phenoxyacetic acid in the Standard solution (mg/mL)
CU = concentration of Penicillin V in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.5%
4.2 Limit of p-Hydroxypenicillin V
Mobile phase, System suitability solution, Standard solution, Sample solution, Chromatographic system and System suitability: Proceed as directed in the Assay.
Analysis
Sample: Sample solution
Calculate the percentage of p-hydroxypenicillin V in the portion of Penicillin V taken:
Result = (rU /rT ) × 100
rU = peak response of p-hydroxypenicillin V from the Sample solution
rT = sum of the peak responses of p-hydroxypenicillin V and penicillin V
Acceptance criteria: NMT 5.0%
5 SPECIFIC TESTS
Crystallinity 〈695〉: Meets the requirements
pH 〈791〉
Sample solution: Prepare a suspension containing 30 mg/mL of Penicillin V in water
Acceptance criteria: 2.5–4.0
Water Determination 〈921〉, Method I: NMT 2.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
Labeling: Label it to indicate that it is to be used in the manufacture of nonparenteral drugs only.
USP Reference Standards 〈11〉
USP Penicillin G Potassium RS
USP Penicillin V RS
USP Penicillin V Potassium RS


