Penicillin G Sodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
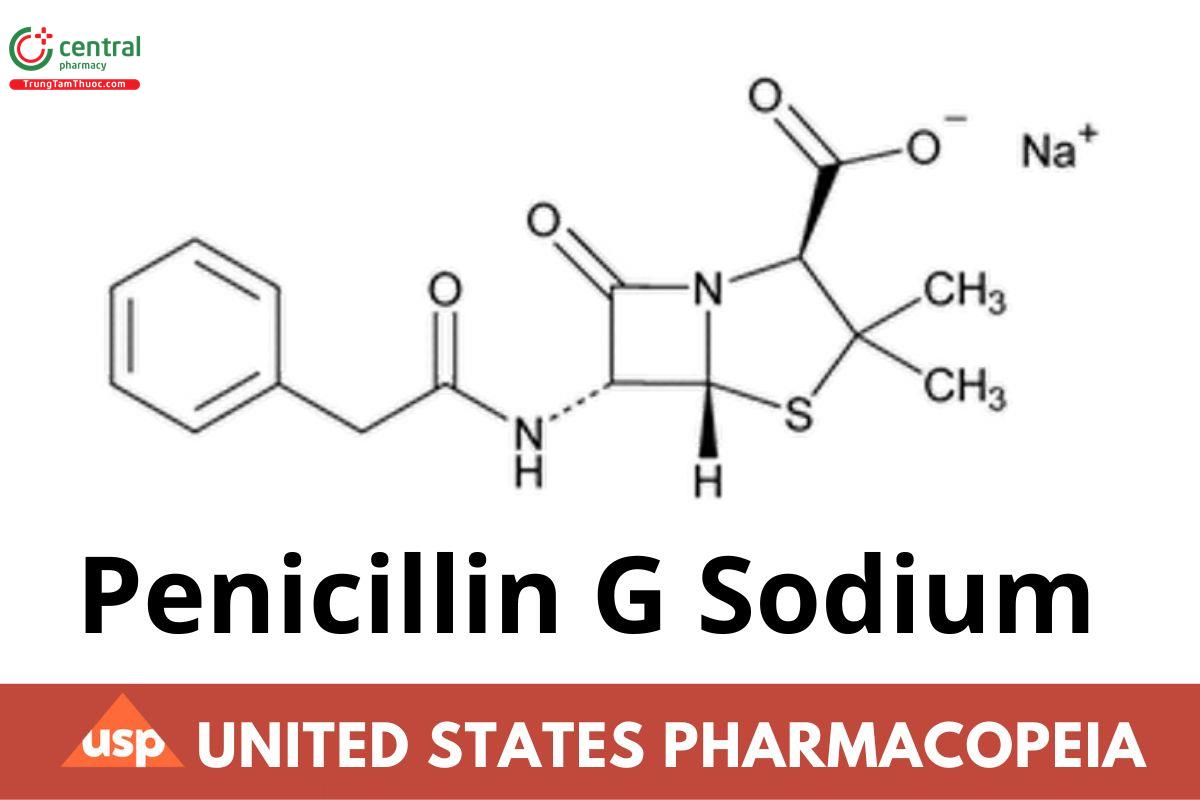
C16H17N2NaO4S 356.37
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-7-oxo-6-[(phenylacetyl)amino]-, [2S-(2α,5α,6β)]-, monosodium salt; Monosodium (2S,5R,6R)-3,3-dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate CAS RN®: 69-57-8; UNII: YS5LY7JF4N.
1 DEFINITION
Penicillin G Sodium has a potency of NLT 1500 and NMT 1750 Penicillin G Units/mg.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B. Identification Tests—General 〈191〉, Sodium: Meets the requirements
3 ASSAY
Procedure
Solution A: 0.01 M monobasic potassium phosphate
Mobile phase: Methanol and Solution A (40:60)
System suitability solution: 0.1 mg/mL each of USP Penicillin G Potassium RS and 2-phenylacetamide in water Standard solution: 0.1 mg/mL of USP Penicillin G Potassium RS in water. Shake as needed to dissolve. This solution contains about 160 Penicillin G Units/mL.
Sample solution: 0.1 mg/mL of Penicillin G Sodium in water
3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 10-cm; 5-µm packing L1
Flow rate: 1 mL/min
Injection volume: 10 µL
3.2 System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for 2-phenylacetamide and penicillin G are about 0.8 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.0 between 2-phenylacetamide and penicillin G, System suitability solution
Column efficiency: NLT 1000 theoretical plates, Standard solution
Tailing factor: NMT 2.0, Standard solution
Relative standard deviation: NMT 2.0%, Standard solution
3.3 Analysis
Samples: Standard solution and Sample solution
Calculate the potency of penicillin G sodium, in Penicillin G Units/mg, in the portion of Penicillin G Sodium taken:
Result = (rU /rS) × (C /CU ) × P
rU = peak response of penicillin G from the Sample solution
rS = peak response of penicillin G from the Standard solution
CS = concentration of USP Penicillin G Potassium RS in the Standard solution (mg/mL)
CU = concentration of Penicillin G Sodium in the Sample solution (mg/mL)
P = potency of penicillin G in USP Penicillin G Potassium RS (Penicillin G Units/mg)
Acceptance criteria: 1500–1750 Penicillin G Units/mg
4 SPECIFIC TESTS
Crystallinity 〈695〉: Meets the requirements
pH 〈791〉
Sample solution: 60 mg/mL of Penicillin G Sodium in water
Acceptance criteria: 5.0–7.5
Loss on Drying 〈731〉
Sample: 100 mg of Penicillin G Sodium
Analysis: Dry the Sample in a capillary-stoppered bottle under a vacuum at a pressure NMT 5 mm of mercury at 60° for 3 h. Acceptance criteria: NMT 1.5%
Sterility Tests 〈71〉: Where the label states that Penicillin G Sodium is sterile, it meets the requirements when tested as directed in Test for Sterility of the Product to Be Examined, Membrane Filtration.
Bacterial Endotoxins Test 〈85〉: Where the label states that Penicillin G Sodium is sterile or it must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.01 USP Endotoxin Units/100 Penicillin G Units.
5 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
Labeling: Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP Reference Standards 〈11〉
USP Penicillin G Potassium RS
USP Penicillin G Sodium RS


