Pemetrexed Disodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
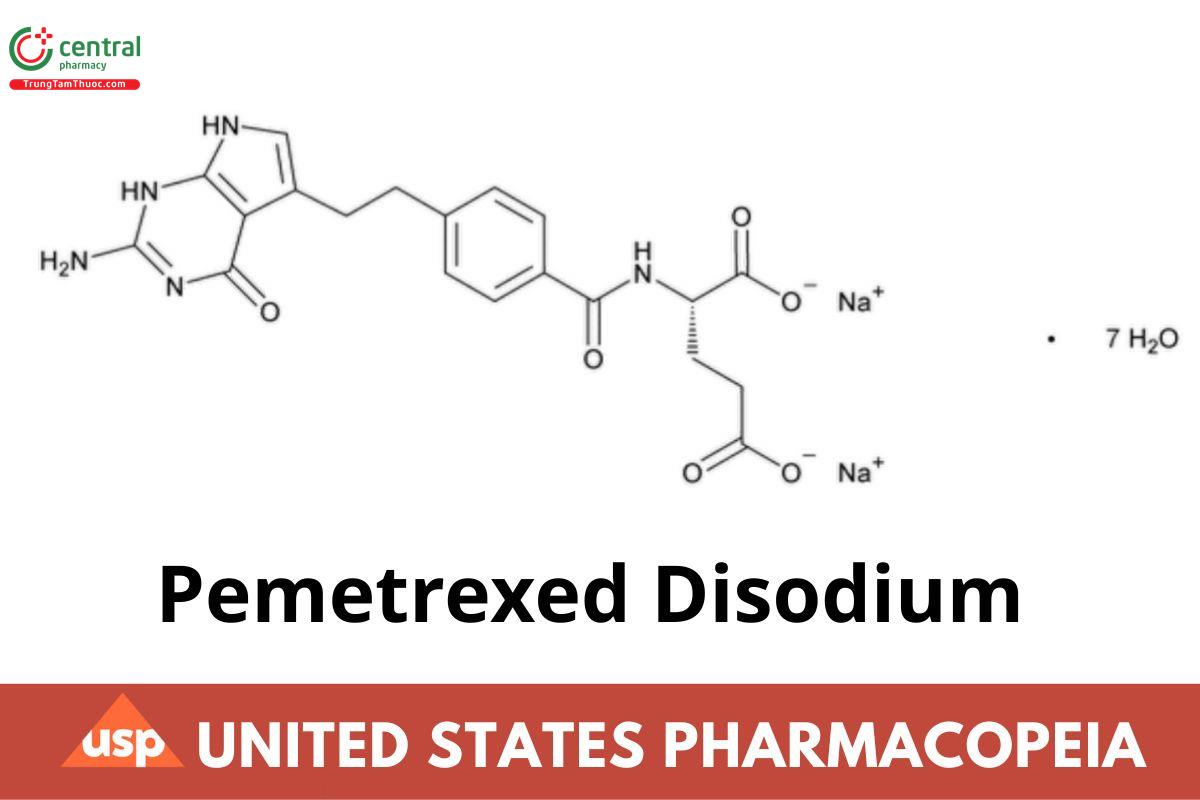
C20H19N5Na2O6 · 7H2O 597.49
l-Glutamic acid, N-[4-[2-(2-amino-4,7-dihydro-4-oxo-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-, disodium salt, heptahydrate; Disodium N-{p-[2-(2-amino-4,7-dihydro-4-oxo-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-l-glutamate heptahydrate CAS RN®: 357166-29-1; UNII: 9T47E4OM16. (RB 7-Aug-2024)
C20H19N5Na2O6 471.38
Anhydrous CAS RN®: 150399-23-8; UNII: 2PKU919BA9 (RB 7-Aug-2024)
C20H21N5O6 427.42
Pemetrexed (free acid) CAS RN®: 137281-23-3; UNII: 04Q9AIZ7NO.
C20H19N5Na2O6 · 2.5 H2O 516.42
Hemipentahydrate CAS RN®: 357166-30-4; UNII: F4GSH45R4C. (RB 7-Aug-2024)
1 DEFINITION
Pemetrexed Disodium contains NLT 97.5% and NMT 102.0% of pemetrexed disodium (C20H19N5Na2O6), calculated on the anhydrous and solvent-free basis.
[Caution—Handle pemetrexed disodium with great care as it alters genetic material and may be irritating to the eyes and skin.]
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A or 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Enantiomeric Purity test.
C. Identification Tests—General 〈191〉, Chemical Identification Tests, Sodium
3 ASSAY
Procedure
Buffer: 0.17% (v/v) glacial acetic acid in water. Adjust with a 50% sodium hydroxide solution to a pH of 5.3 ± 0.1.
Mobile phase: Acetonitrile and Buffer (11:89)
Standard solution: 0.15 mg/mL of USP Pemetrexed Disodium RS in water
Sample solution: 0.15 mg/mL of Pemetrexed Disodium in water
3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 285 nm
Column: 4.6-mm × 7.5-cm; 3.5-µm packing L7
Column temperature: 30°
Injection volume: 20 µL
3.2 System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: 0.8–1.5
Relative standard deviation: NMT 0.73%
3.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of pemetrexed disodium (C20H19N5Na2O6) in the portion of Pemetrexed Disodium taken:
Result = (rU/rS) × (CS /CU ) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Pemetrexed Disodium RS in the Standard solution (mg/mL)
CU = concentration of Pemetrexed Disodium in the Sample solution (mg/mL)
Acceptance criteria: 97.5%–102.0% on the anhydrous and solvent-free basis
4 IMPURITIES
4.1 Organic Impurities
Buffer: 1.45 g/L of ammonium formate in water. Adjust with formic acid to a pH of 3.5 ± 0.1.
Solution A: Acetonitrile and Buffer (5:95)
Solution B: Acetonitrile and Buffer (30:70)
Mobile phase: See Table 1. [Note—After each injection, re-equilibrate the chromatographic system at the initial condition for a minimum of 13 min.]
Table 1
Time (min) | Solution A (%) | Solution B (%) |
0 | 100 | 0 |
45 | 0 | 100 |
47 | 100 | 0 |
System suitability stock solution: Prepare 3 mg/mL of USP Pemetrexed Disodium RS in 0.1 N sodium hydroxide. Heat this solution at 70° for 40 min.
[Note—The preparation degrades pemetrexed and generates the pemetrexed R-dimer and pemetrexed S-dimer as follows: Pemetrexed R-dimer: (2S,2′S)-2,2′-{[(R)-2,2′-Diamino-4,4′,6-trioxo-1,4,4′,6,7,7′-hexahydro-1′H,5H-5,6′-bipyrrolo[2,3-d]pyrimidine-5,5′- diyl]bis(ethylenebenzene-4,1-diylcarbonylimino)}diglutaric acid.
Pemetrexed S-dimer: (2S,2′S)-2,2′-{[(S)-2,2′-Diamino-4,4′,6-trioxo-1,4,4′,6,7,7′-hexahydro-1′H,5H-5,6′-bipyrrolo[2,3-d]pyrimidine-5,5′- diyl]bis(ethylenebenzene-4,1-diylcarbonylimino)}diglutaric acid.]
System suitability solution: Transfer 1 mL of the System suitability stock solution to a 10-mL volumetric ask and dilute with water to volume. Sensitivity solution: 0.1 µg/mL of USP Pemetrexed Disodium RS in water
Sample solution: 0.2 mg/mL of Pemetrexed Disodium in water. Do not sonicate.
4.1.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 250 nm
Column: 4.6-mm × 15-cm; 3.5-µm packing L7
Autosampler temperature: 2°–8°
Flow rate: 1 mL/min
Injection volume: 20 µL
4.1.2 System suitability
Samples: System suitability solution and Sensitivity solution
[Note—The relative retention times for the pemetrexed R-dimer and pemetrexed S-dimer peaks are 0.87 and 0.88, respectively.]
Suitability requirements
Peak-to-valley ratio: The ratio of the height of the pemetrexed R-dimer peak to the height of the valley between the pemetrexed R-dimer and pemetrexed S-dimer is NLT 1.5, System suitability solution.
Signal-to-noise ratio: NLT 10, Sensitivity solution
4.1.3 Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Pemetrexed Disodium taken:
Result = (rU /rT ) × 100
rU = peak area of each impurity from the Sample solution
rT = total peak areas from the Sample solution
Acceptance criteria: See Table 2. Disregard any peak less than 0.05%.
Table 2
Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
N-Methyl pemetrexeda | 0.82 | 0.15 |
Pemetrexed glutamideb | 0.90 | 0.15 |
Pemetrexed | 1.0 | — |
Any individual unspecified impurity | — | 0.10 |
Total impurities | — | 0.60 |
a {4-[2-(2-Amino-1-methyl-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-l-glutamic acid.
b {4-[2-(2-Amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-4-l-glutamyl-l-glutamic acid.
4.2 Enantiomeric Purity
Buffer: Dissolve 8 g of anhydrous beta cyclodextrin in 1 L of water. Add 15 mL of triethylamine to this solution and mix. Add about 6 mL of phosphoric acid and adjust with additional phosphoric acid to a pH of 6.0.
Mobile phase: Acetonitrile and Buffer (5:95)
Standard solution: 0.24 mg/mL of USP Pemetrexed Disodium RS in water
Sensitivity solution: 0.12 µg/mL of USP Pemetrexed Disodium RS in water from the Standard solution
Sample solution: 0.24 mg/mL of Pemetrexed Disodium in water
4.2.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 230 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Temperatures
Autosampler: 2°–8°
Column: 40°
Flow rate: 1 mL/min
Injection volume: 50 µL
4.2.2 System suitability
Samples: Standard solution and Sensitivity solution
[Note—USP Pemetrexed Disodium RS contains a small amount of pemetrexed enantiomer disodium (disodium N-{p-[2-(2-amino-4,7-dihydro 4-oxo-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-d-glutamate). The relative retention times for pemetrexed enantiomer and pemetrexed are about 0.94 and 1.0, respectively.]
Suitability requirements
Peak-to-valley ratio: The ratio of the height of the pemetrexed enantiomer peak to the height of the valley between the pemetrexed enantiomer and pemetrexed is NLT 5.0, Standard solution
Signal-to-noise ratio: NLT 10 for the pemetrexed peak, Sensitivity solution
4.2.3 Analysis
Sample: Sample solution
Calculate the percentage of pemetrexed enantiomer in the portion of Pemetrexed Disodium taken:
Result = (rU /rT ) × 100
rU = peak area of pemetrexed enantiomer from the Sample solution
rT = total peak areas of pemetrexed enantiomer and pemetrexed from the Sample solution
Acceptance criteria: NMT 0.3%
5 SPECIFIC TESTS
Change to read:
Water Determination 〈921〉, Method I, Method Ia or Method Ic:
For heptahydrate form: (RB 7-Aug-2024) 19.5%–22.1%
For hemipentahydrate form: 8.0%–10.5% (RB 7-Aug-2024)
pH 〈791〉
Sample: 56 mg/mL in water
Acceptance criteria: 7.5–8.4
Bacterial Endotoxins Test 〈85〉: It contains less than 0.17 USP Endotoxin Units/mg of pemetrexed.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. Store at room temperature.
Add the following:
Labeling: Label to indicate where it is hemipentahydrate form. (RB 7-Aug-2024)
Change to read:
USP Reference Standards 〈11〉
(RB 7-Aug-2024)
USP Pemetrexed Disodium RS


