Padimate O
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
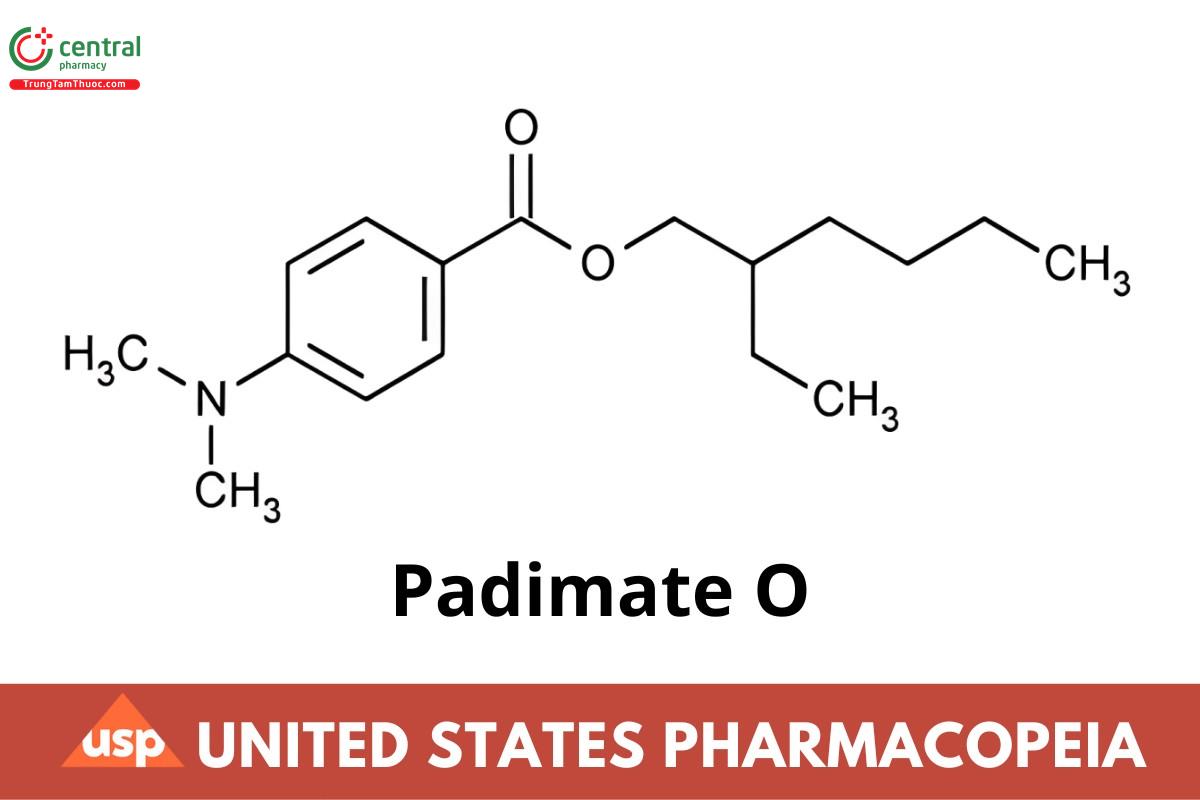
C17H27NO2 277.40
Benzoic acid, 4-(dimethylamino)-, 2-ethylhexyl ester,
2-Ethylhexyl p-(dimethylamino)benzoate CAS RN®: 21245-02-3; UNII: Z11006CMUZ.
1 DEFINITION
Padimate O contains NLT 97.0% and NMT 102.0% of padimate O (C17H27NO2).
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197F (CN 1-MAY-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 PROCEDURE
Mobile phase: Methanol, water, and glacial acetic acid (850:150:0.5)
Standard solution: 0.1 mg/mL of USP Padimate O RS in methanol
Sample solution: 0.1 mg/mL of Padimate O in methanol
3.2 Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 308 nm
Column: 4.6-mm x 15-cm; 5-µm packing L1
Flow rate: 1.5 mL/min
Injection volume: 10 µL
3.3 System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 0.73%
3.4 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of padimate O (C17H27NO2) in the portion of Padimate O taken:
Result = (rU/rS) x (CS/CU) x 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Padimate O RS in the Standard solution (mg/mL)
CU = concentration of Padimate O in the Sample solution (mg/mL)
Acceptance criteria: 97.0%-102.0%
4 IMPURITIES
ORGANIC IMPURITIES
Sample solution: 10 mg/mL of Padimate O in chloroform
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 3-mm x 1.8-m stainless steel packed with 10% liquid phase G9 on support S1A
Carrier gas: Helium
Column temperature: See Table 1.
Table 1
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 150 | 10 | 250 | 10 |
Injection volume: 2 µL
Analysis
Sample: Sample solution
Calculate the percentage of the total impurities in the portion of Padimate O taken:
[ΣrI/(ΣrI + rU)] x 100
rI = individual impurity peak response from the Sample solution
rU = Padimate O peak response from the Sample solution
Acceptance criteria
Total impurities: NMT 2.0%
5 SPECIFIC TESTS
5.1 SPECIFIC GRAVITY (841)
0.990-1.000
5.2 REFRACTIVE INDEX (831)
1.5390-1.5430
5.3 FATS AND FIXED OILS, Acid Value (401)
NMT 1.0
5.4 FATS AND FIXED OILS, Saponification Value(401)
Analysis: Proceed as directed in the chapter, except to maintain reflux for 4 h.
Acceptance criteria: 195-215
6 ADDITIONAL REQUIREMENTS
6.1 PACKAGING AND STORAGE
Preserve in tight, light-resistant containers.
6.2 USP REFERENCE STANDARDS (11)
USP Padimate O RS


