Paclitaxel
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
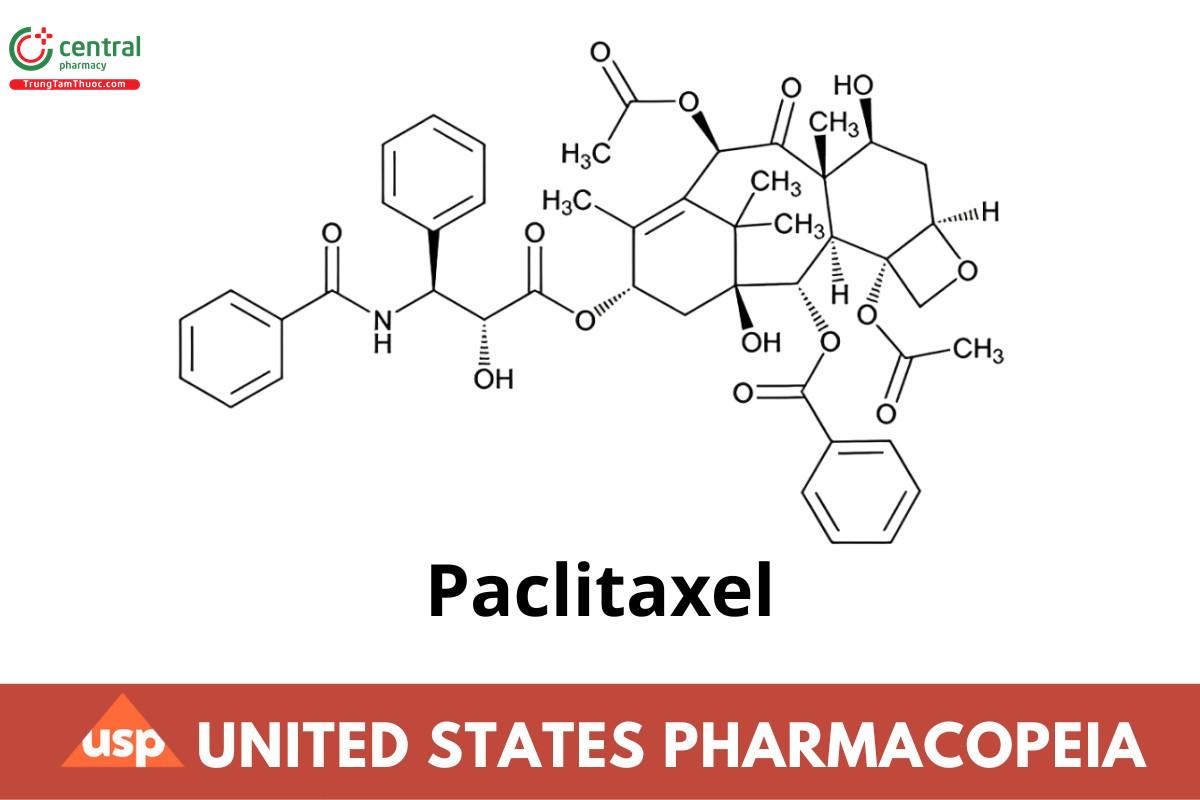
C47H51NO14 853.92
Benzenepropanoic acid, β-(benzoylamino)-α-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, [2aR-[2aα, 4β,4aβ,6β,9α(αR*,βS*),11α,12α,12aα,12bα]];
(2aR,4S,4aS,6R,9S,115,125, 12aR,12bS)-1,2a,3,4,4a,6,9,10,11,12,12a, 12b-Dodecahydro-4,6,9,11,12,12b-hexahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca[3,4]-benz[1,2-b]oxet-5-one 6,12b-diacetate, 12-benzoate, 9-ester with (2R,3S)-N-benzoyl-3-phenylisoserine; (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-9-{[(2R,3S)-3-Benzamido-2-hydroxy-3-phenylpropanoyl]oxy}-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate CAS RN®: 33069-62-4; UNII: P88XT4IS4D.
1 DEFINITION
Paclitaxel contains NLT 97.0% and NMT 102.0% of paclitaxel (C47H51NO14), calculated on the anhydrous and solvent-free basis.
[CAUTION-Paclitaxel is cytotoxic. Great care should be taken to prevent inhaling particles of Paclitaxel and exposing the skin to it.]
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 PROCEDURE
Diluent: Methanol and acetic acid (200:1)
Mobile phase: Acetonitrile and water (45:55)
Standard solution: 1 mg/mL of USP Paclitaxel RS in Diluent. Sonicate to dissolve if necessary.
Sample solution: 1 mg/mL of Paclitaxel in Diluent. Sonicate to dissolve if necessary.
3.2 Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 227 nm
Column: 4.6-mm x 25-cm; 5-µm packing L43
Flow rate: 1.5 mL/min
Injection volume: 10 µL
3.3 System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: 0.7-1.3
Relative standard deviation: NMT 1.5%
3.4 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of paclitaxel (C47H51NO14) in the portion of Paclitaxel taken:
Result = (rU/rS) x (CS/CU) x 100
rU = peak response of paclitaxel from the Sample solution
rS = peak response of paclitaxel from the Standard solution
CS = concentration of USP Paclitaxel RS in the Standard solution (mg/mL)
CU = concentration of Paclitaxel in the Sample solution (mg/mL)
Acceptance criteria: 97.0%-102.0%, on the anhydrous and solvent-free basis
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281)
NMT 0.2%
Change to read:
4.2 ORGANIC IMPURITIES
4.2.1 Test 1
(for material labeled as isolated from natural sources): If the material complies with this test, the labeling indicates that it meets the requirements of USP Organic Impurities Test 1.
Solution A: Acetonitrile
Solution B: Water
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 35 | 65 |
| 35 | 35 | 65 |
| 60 | 80 | 20 |
| 70 | 35 | 65 |
| 80 | 35 | 65 |
Diluent: Methanol and acetic acid (200:1)
System suitability stock solution: 10 µg/mL each of USP Paclitaxel Related Compound A RS and USP Paclitaxel Related Compound B RS in methanol
System suitability solution: 1 µg/mL each of USP Paclitaxel Related Compound A RS and USP Paclitaxel Related Compound B RS from System suitability stock solution in Diluent
Standard solution: 5 µg/mL of USP Paclitaxel RS in Diluent. Sonicate to dissolve if necessary.
Sample solution: 1 mg/mL of Paclitaxel in Diluent. Sonicate to dissolve if necessary.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 227 nm
Column: 4.6-mm x 25-cm; 5-µm packing L43
Column temperature: 30°
Flow rate: 2.6 mL/min
Injection volume: 15 µL
System suitability
Samples: System suitability solution and Standard solution
[NOTE-See Table 2 for the relative retention times.]
Suitability requirements
Resolution: NLT 1.0 between paclitaxel related compound A and paclitaxel related compound B, System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Sample: Sample solution
Calculate the percentage of each specified and unspecified impurity in the portion of Paclitaxel taken:
Result = (rU/rS) x 1/F x 100
rU = peak response of each individual impurity
rS = peak response of paclitaxel
F = relative response factor for each individual impurity (see Table 2)
Acceptance criteria: See Table 2.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Baccatin IIIa | 0.24 | 0.78 | 0.2 |
| 10-Deacetylpaclitaxeb | 0.53 | 1.00 | 0.5 |
| 7-Xylosylpaclitaxelc | 0.57 | 1.00 | 0.2 |
| Paclitaxel related compound A | 0.78 | 0.79 | a1d |
| Paclitaxel sec-Butyl analoge | 0.78 | 0.79 | a2d |
| Paclitaxel related compound B | 0.86 | 1.00 | 0.5 |
| Paclitaxel | 1.00 | — | — |
| Paclitaxel benzyl analogf | 1.10 | 1.00 | b1g |
| 3′′,4′′-Dehydro paclitaxel Ch | 1.10 | 1.00 | b2g |
| 7-Epicephalomanninei | 1.40 | 1.00 | 0.3 |
| 7-Epipaclitaxelj | 1.85 | 1.00 | 0.5 |
| Any unspecified impurity | — | 1.00 | 0.1 |
| Total impurities | — | — | 2.0 |
a (2aR,4S, 4aS,6R,9S,11S,12S,12aR,12bS)-12-(Benzoyloxy)-4,9,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4] benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
b (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-Acetoxy-9-{[(2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl]oxy}-4,6,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-12-yl benzoate.
c (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-9-{[(2R,3S)-3-Benzamido-2-hydroxy-3-phenylpropanoyl]oxy}-12-(benzoyloxy)-11-hydroxy-4a,8,13,13-tetramethyl-5-oxo-4-(D-xylopyranosyloxy)-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca [3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
d Resolution may be incomplete for these peaks depending upon the relative amounts present; the sum of a1 and a2 is NMT 0.5%.
e 2",3"-Dihydrocephalomannine;(2aR,4S, 4aS,6R,9S,11S,12S,12aR,12bS)-12-(Benzoyloxy)-4,11-dihydroxy-9-{[(2R,3S)-2-hydroxy-3-(2-methylbutanamido)-3-phenylpropanoyl]oxy}-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca [3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
f (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12-(Benzoyloxy)-4,11-dihydroxy-9-{[(2R,3S)-2-hydroxy-3-phenyl-3-(2-phenylacetamido)propanoyl]oxy}-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca [3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
g Resolution may be incomplete for these peaks depending upon the relative amounts present; the sum of b1 and b2 is NMT 0.5%.
h (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12-(Benzoyloxy)-9-({(2R,3S)-3-[(Z)-hex-3-enamido]-2-hydroxy-3-phenylpropanoyl}oxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
i (2aR,4R,4aS,6R,9S,11S,12S,12aR,12bS)-12-(Benzoyloxy)-4,11-dihydroxy-9-({(2R,3S)-2-hydroxy-3-[(E)-2-methylbut-2-enamido]-3-phenylpropanoyl}oxy)-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
j (2aR,4R,4aS,6R,9S,11S,12S,12aR,12bS)-9-{[(2R,3S)-3-Benzamido-2-hydroxy-3-phenylpropanoyl]oxy}-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
4.2.2 Test 2
(for material labeled as produced by a semisynthetic process): If the material complies with this test, the labeling indicates that it meets the requirements of USP Organic Impurities Test 2.
Solution A: Acetonitrile and water (40:60)
Solution B: Acetonitrile
Mobile phase: See Table 3.
Table 3
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 20 | 100 | 0 |
| 60 | 10 | 90 |
| 62 | 100 | 0 |
| 70 | 100 | 0 |
System suitability solution: 0.96 mg/mL of USP Paclitaxel RS and 0.008 mg/mL of USP Paclitaxel Related Compound B RS in Solution B. Sonicate to dissolve if necessary.
Sample solution: 1 mg/mL of Paclitaxel in Solution B. Sonicate to dissolve if necessary.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 227 nm
Column: 4.6-mm x 15-cm; 3-µm packing L1
Column temperature: 35°
Flow rate: 1.2 mL/min
Injection volume: 15 µL
System suitability
Sample: System suitability solution
[NOTE-See Table 4 for the relative retention times.]
Suitability requirements
Resolution: NLT 1.2 between paclitaxel related compound B and paclitaxel
Relative standard deviation: NMT 2.0% for paclitaxel
Analysis
Sample: Sample solution
Calculate the percentage of each specified and unspecified impurity in the portion of Paclitaxel taken:
Result = (rU/rT) x 1/F x 100
rU = peak response of each individual impurity from the Sample solution
rT = sum of all the peak responses from the Sample solution
F = relative response factor for each impurity (see Table 4)
Acceptance criteria: See Table 4.
Table 4
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| 10-Deacetylbaccatin IIIa | 0.11 | 0.81 | 0.1 |
| Baccatin IIIb | 0.20 | 0.78 | 0.2 |
| Paclitaxel photodegradantc | 0.42 | 0.72 | 0.1 |
| 10-Deacetylpaclitaxeld | 0.47 | 1.00 | 0.5 |
| 2-Debenzoylpaclitaxel-2- pentenoatee | 0.80 | 1.00 | 0.7 |
| Oxetane ring opened, acetyl and benzoyl migratedf | 0.92g | 1.00 | x1g |
| 10-Acetoacetylpaclitaxelh | 0.92g | 1.00 | x2g |
| Paclitaxel related compound B | 0.94g | 1.00 | x3g |
| Paclitaxel | 1.00 | — | — |
| 7-Epipaclitaxeli | 1.37 | 1.00 | 0.4 |
| 10,13-Bissidechainpaclitaxelj | 1.45 | 1.00 | 0.5 |
| 7-Acetylpaclitaxelk | 1.54 | 1.00 | 0.6 |
| 13-O-(Triethylsilyl)baccatin IIIl | 1.80 | 0.57 | 0.1 |
| 7-O-(Triethylsilyl)paclitaxelm | 2.14 | 1.00 | 0.3 |
| Any unspecified impurity | — | 1.00 | 0.1 |
| Total impurities | — | — | 2.0 |
a (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-(Acetyloxy)-12-(benzoyloxy)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-dodecahydro-4,6,9,11-tetrahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca[3,4]benz[1,2-b]oxet-5-one.(ERR 1-Oct-2024)
b (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12-(Benzoyloxy)-4,9,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4] benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
c (1S,2S,4S,5S,5aR,6R,7aS,8S,9aR,11aS,11bS)-4-{[(2R,3S)-3-Benzamido-2-hydroxy-3-phenylpropanoyl]oxy}-1-(benzoyloxy)-2,8-dihydroxy-5,7a,12,12-tetramethyl-7-oxodecahydro-1H-2,5a-methanocyclohepta[3,3a]indeno[5,4-b]oxete-6,11a(11H)-diyl diacetate.
d (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-Acetoxy-9-{[(2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl]oxy}-4,6,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-12-yl benzoate.
e (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-9-{[(2R,3S)-3-Benzamido-2-hydroxy-3-phenylpropanoyl]oxy}-4,11-dihydroxy-4a,8,13,13-tetramethyl-12-{[(E)-2-methylbut-2-enoyl]oxy}-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
f (1S,3S,4S,4aR,5S,6S,8S,11R,12aS)-8-{[(2R,3S)-3-Benzamido-2-hydroxy-3-phenylpropanoyl] oxy}-4-[(benzoyloxy)methyl]-1,4,5,6-tetrahydroxy-9,12a,13,13-tetramethyl-12-oxo-1,2,3,4,4a,5,6,7,8,11,12,12a-dodecahydro-6,10-methanobenzocyclodecen-3,11-diyl diacetate.
g Resolution may be incomplete for these peaks depending upon the relative amounts present; the sum of x1, x2, and x3 is NMT 0.4%.
h (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6-Acetoxy-9-{[(2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl]oxy}-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-12b-[(3-oxobutanoyl)oxy]-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-bloxet-12-yl benzoate.
i (2aR, 4R,4aS,6R,9S,11S,12S,12aR,12bS)-9-{[(2R,3S)-3-Benzamido-2-hydroxy-3-phenylpropanoyl]oxy}-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
j (2aR,4S, 4aS,6S,9S,11S,12S,12aR,12bS)-12b-Acetoxy-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,9-diyl (2R,2'R,3S,3'S)-bis(3-benzamido-2-hydroxy-3-phenylpropanoate).
k (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-9-{[(2R,3S)-3-Benzamido-2-hydroxy-3-phenylpropanoyl]oxy}-12-(benzoyloxy)-11-hydroxy-4a,8,13,13-tetramethyl-5-oxo-4-(acetyloxy)-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
l 13-Tes-baccatin III; (2aR,4S, 4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-(Diacetyloxy)-12-(benzoyloxy)-9-(triethylsilyloxy)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca[3,4]benz[1,2-b]oxet-5-one.
m 7-Tes-paclitaxel; (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-9-{[(2R,3S)-3-Benzamido-2-hydroxy-3-phenylpropanoyl]oxy}-12-(benzoyloxy)-4-(triethylsilyloxy)-11-hydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
4.2.3 Test 3
(for material labeled as produced by a plant cell fermentation process): If the material complies with this test, the labeling indicates that it meets the requirements of USP Organic Impurities Test 3.
Solution A: Acetonitrile and water (40:60)
Solution B: Acetonitrile
Mobile phase: See Table 5.
Table 5
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 28 | 100 | 0 |
| 33 | 98 | 2 |
| 58 | 10 | 90 |
| 60 | 10 | 90 |
| 63 | 100 | 0 |
| 70 | 100 | 0 |
System suitability solution: 1 mg/mL of USP Paclitaxel Impurity Mixture RS in Solution B. Sonicate to dissolve if necessary.
Standard solution: 1 mg/mL of USP Paclitaxel RS in Solution B. Sonicate to dissolve if necessary.
Sample solution: 1 mg/mL of Paclitaxel in Solution B. Sonicate to dissolve if necessary.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 227 nm
Column: 4.6-mm x 15-cm; 3-µm packing L1
Flow rate: 1.2 mL/min
Injection volume: 12 µL
System suitability
Samples: System suitability solution and Standard solution
[NOTE-See Table 6 for the relative retention times.]
Suitability requirements
Resolution: NLT 1.8 between paclitaxel and paclitaxel benzyl analog, System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Sample: Sample solution
Calculate the percentage of each specified and unspecified impurity in the portion of Paclitaxel taken:
Result = (rU/rT) x 100
rU = peak response of each individual impurity from the Sample solution
rT = sum of all the peak responses from the Sample solution
Acceptance criteria: See Table 6.
Table 6
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Paclitaxel propyl analoga | 0.54 | 0.2 |
| Paclitaxel related compound A | 0.76 | 0.5 |
| Paclitaxel sec-Butyl analogb | 0.81 | 0.2 |
| Paclitaxel n-Butyl analogc | 0.89 | 0.1 |
| Paclitaxel | 1.00 | — |
| Paclitaxel benzyl analogd | 1.10 | 0.4 |
| Baccatin VIe | 1.23 | 0.2 |
| Paclitaxel pentyl analogf | 1.31 | 0.2 |
| 7-Epipaclitaxelg | 1.51 | 0.4 |
| Any unspecified impurity | — | 0.1 |
| Total impurities | — | 2.0 |
a (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12-(Benzoyloxy)-9-{[(2R,3S)-3-butyramido-2-hydroxy-3-phenylpropanoyl)oxy}-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
b 2",3"-Dihydrocephalomannine; (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12-(Benzoyloxy)-4,11-dihydroxy-9-{[(2R,3S)-2-hydroxy-3-(2-methylbutanamido)-3-phenylpropanoyl)oxy}-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
c (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12-(Benzoyloxy)-4,11-dihydroxy-9-{[(2R,3S)-2-hydroxy-3-pentanamido-3-phenylpropanoyl]oxy}-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
d (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12-(Benzoyloxy)-4,11-dihydroxy-9-{[(2R,3S)-2-hydroxy-3-phenyl-3-(2-phenylacetamido)propanoyl]oxy}-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
e (2aR,4S, 4aS,5R,6R,9S,11S,12S,12aR,12bS)-12-(Benzoyloxy)-11-hydroxy-4a,8,13,13-tetramethyl-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-4,5,6,9,12b(2aH)-pentayl pentaacetate.
f (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12-(Benzoyloxy)-9-{[(2R,3S)-3-hexanamido-2-hydroxy-3-phenylpropanoyl]oxy}-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
g (2aR,4R,4aS,6R,9S,11S,12S,12aR,12bS)-9-{[(2R,3S)-3-Benzamido-2-hydroxy-3-phenylpropanoyl]oxy}-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate.
5 SPECIFIC TESTS
5.1 MICROBIAL ENUMERATION TESTS (61) and TESTS FOR SPECIFIED MICROORGANISMS (62)
The total aerobic microbial count does not exceed 102 cfu/g. It meets the requirements of the tests for absence of Salmonella species, Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa.
5.2 OPTICAL ROTATION (781S), Procedures, Specific Rotation
Sample solution: 10 mg/mL of paclitaxel in methanol
Acceptance criteria: -49.0° to 55.0° at 20°, calculated on the anhydrous, solvent-free basis
5.3 WATER DETERMINATION (921), Method I, Method Ic
NMT 4.0%
5.4 BACTERIAL ENDOTOXINS TEST (85)
Where the label states Paclitaxel must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins are such that the requirement under the relevant dosage form monograph(s) in which Paclitaxel is used can be met.
6 ADDITIONAL REQUIREMENTS
6.1 PACKAGING AND STORAGE
Preserve in tight, light-resistant containers, and store at controlled room temperature.
6.2 LABELING
Label it to indicate the type of process used to produce the material and the Organic Impurities test with which the material complies. Where Paclitaxel is intended for use in preparing injectable or other sterile dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable or other sterile dosage forms to ensure acceptable levels of bacterial endotoxins.
6.3 USP REFERENCE STANDARDS (11)
USP Paclitaxel RS
USP Paclitaxel Related Compound A RS
Cephalomannine; (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12-(Benzoyloxy)-4,11-dihydroxy-9-({(2R,3S)-2-hydroxy-3-[(E)-2-methylbut-2-enamido]-3-phenylpropanoyl}oxy)-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca [3,4]benzo[1,2-bloxete-6,12b(2aH)-diyl diacetate.
C45H53NO14 831.91
USP Paclitaxel Related Compound B RS
10-Deacetyl-7-epipaclitaxel; (2aR,4R,4aS,6R,9S,11S,12S,12aR,12bS)-12b-Acetoxy-9-{[(2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl]oxy}-4,6,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-12-yl benzoate.
C45H49NO13 811.88
USP Paclitaxel Impurity Mixture RS
It contains a mixture of the following two compounds:
Paclitaxel;
Paclitaxel benzyl analog: (2aR,4S,4aS, 6R,9S,11S,12S,12aR,12bS)-12-(Benzoyloxy)-4,11-dihydroxy-9-{[(2R,3S)-2-hydroxy-3-phenyl-3-(2-phenylacetamido)propanoyl]oxy}-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-bloxete-6,12b(2aH)-diyl diacetate.
C48H53NO14 867.95


