Oxyquinoline Sulfate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
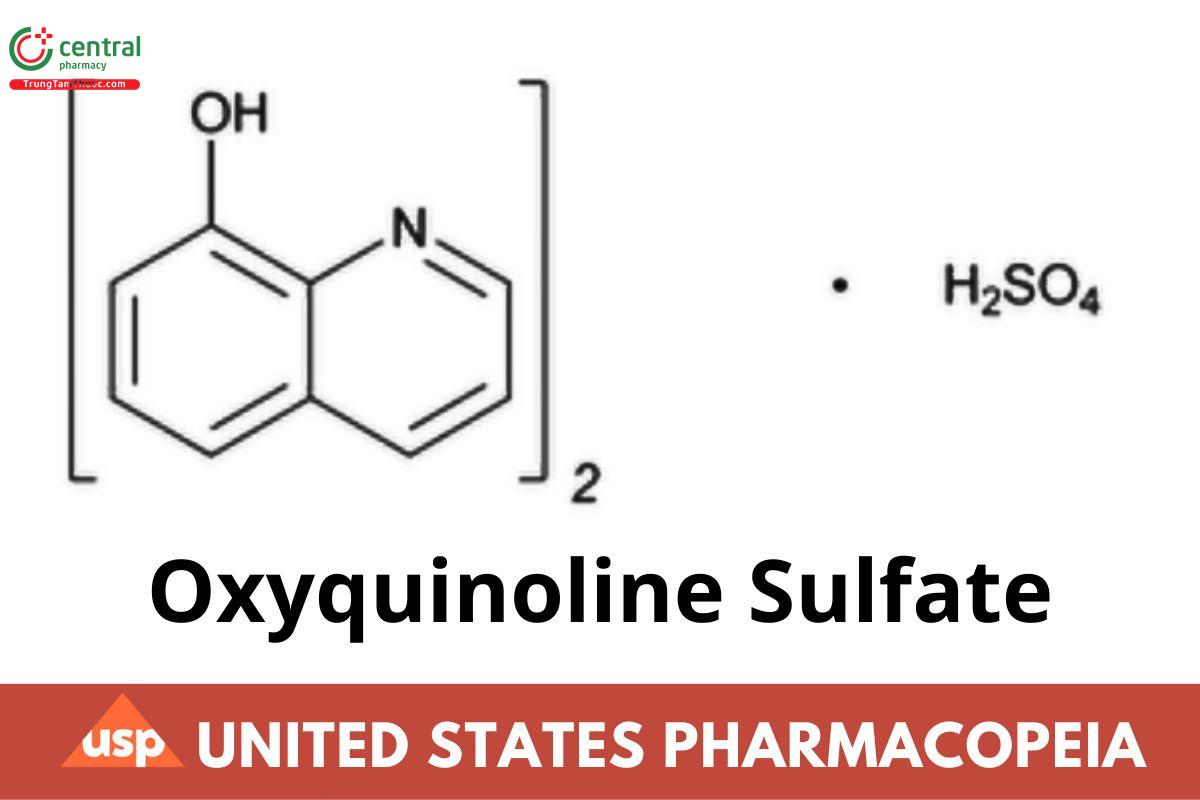
(C9H7NO)2 · H2SO4 388.39
8-Quinolinol sulfate (2:1) (salt) CAS RN®: 134-31-6.
1 DEFINITION
Oxyquinoline Sulfate is 8-hydroxyquinoline sulfate. It contains NLT 97.0% and NMT 101.0% of oxyquinoline sulfate [(C9H7NO)2 · H2SO4], calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M(CN 1-May-2020)
Analysis: On the undried specimen
Acceptance criteria: Meets the requirements
B. Identification Tests—General, Sulfate 〈191〉
Analysis: 100 mg/mL
Acceptance criteria: Meets the requirements
3 ASSAY
Procedure
Sample: 100 mg
Titrimetric system
(See Titrimetry 〈541〉.)
Mode: Residual titration
Titrant: 0.1 N bromine VS
Back-titrant: 0.1 N sodium thiosulfate VS
Endpoint detection: Visual
Analysis: Transfer the Sample to an iodine flask, add 30 mL of glacial acetic acid, 25.0 mL of Titrant, 10 mL of potassium bromide solution (150 mg/mL), and 10 mL of hydrochloric acid. Immediately insert the stopper, mix, and allow to stand for 15 min, protected from light. Quickly add 10 mL of potassium iodide solution (100 mg/mL) and 100 mL of water, taking precautions against the escape of bromine vapor. At once insert the stopper, and shake the mixture thoroughly. Remove the stopper, and rinse it and the neck of the flask with a small quantity of water so that the washing flows into the flask. Shake the mixture thoroughly. Titrate the liberated iodine with Back-titrant, adding 3 mL of starch TS as the endpoint is approached. Perform a blank determination. Each mL of Titrant is equivalent to 4.855 mg of oxyquinoline sulfate [(C9H7NO)2 · H2SO4].
Acceptance criteria: 97.0%–101.0% on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.3%
5 SPECIFIC TESTS
Water Determination, Method I 〈921〉: 4.0%–6.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers.
USP Reference Standards 〈11〉
USP Oxyquinoline Sulfate RS


