Oxymetholone
If you find any inaccurate information, please let us know by providing your feedback here
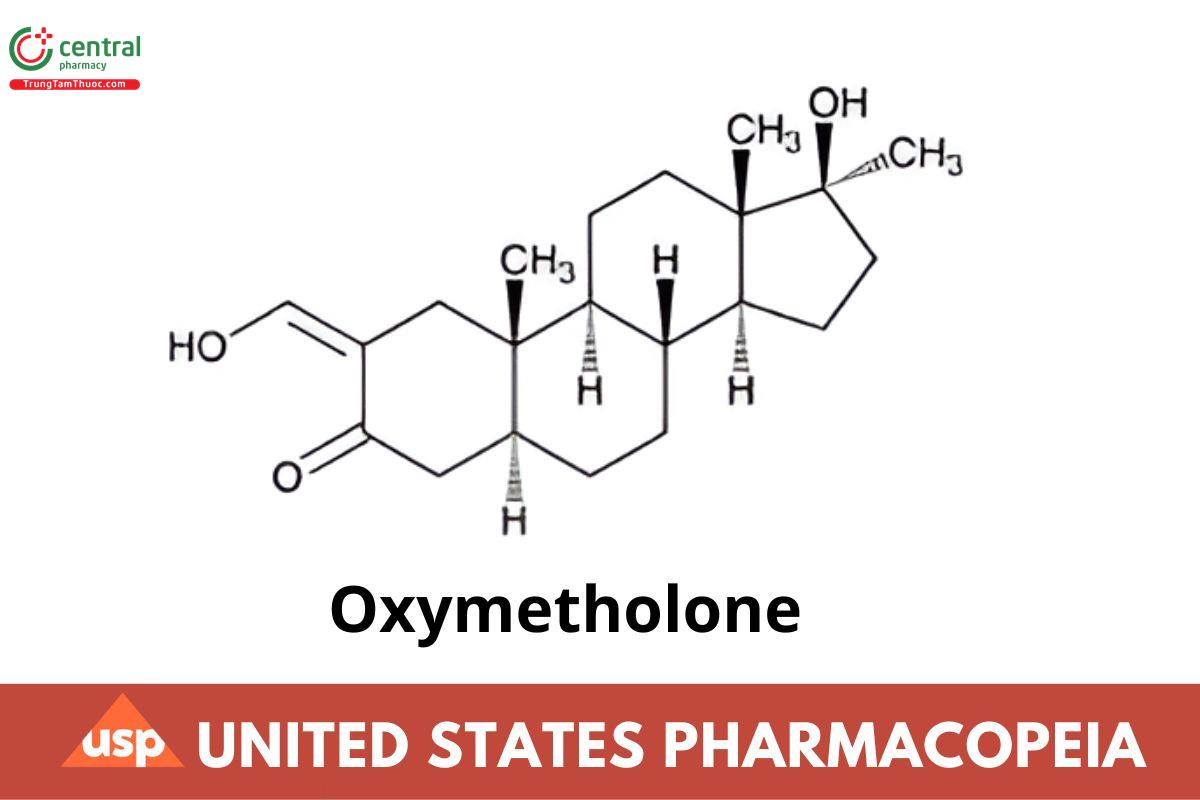
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C21H32O3 332.48
Androstan-3-one, 17-hydroxy-2-(hydroxymethylene)-17-methyl-, (5α,17β)-;
17β-Hydroxy-2-(hydroxymethylene)-17α-methyl-5α-androstan-3-one CAS RN®: 434-07-1; UNII: L76T0ZCA8K.
1 DEFINITION
Oxymetholone contains NLT 97.0% and NMT 103.0% of oxymetholone (C H O ), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K or 197A (USP 1-May-2021)
Delete the following:
B. Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U
Sample solution: 10 μg/mL in 0.01 N methanolic sodium hydroxide
Acceptance criteria: Meets the requirements (USP 1-May-2021)
Add the following:
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. (USP 1-May-2021)
3 ASSAY
Change to read:
Procedure
[Note - Protect solutions containing oxymetholone from light.]
Diluted acetic acid: Dilute 2 mL of glacial acetic acid with 1 L of water.
Mobile phase: Tetrahydrofuran, acetonitrile, and Diluted acetic acid (32:12:56)
Standard solution: 0.1 mg/mL of USP Oxymetholone RS, prepared as follows. Dissolve a suitable amount of the material with 50% of the flask volume of acetonitrile in an appropriate volumetric flask. Sonicate until completely dissolved. Dilute with water to volume.
Sample solution: 0.1 mg/mL of Oxymetholone, prepared as directed in the Standard solution
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.6-mm × 25-cm; 5-μm packing L1
Flow rate: 1.2 mL/min
Injection volume: 20 μL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 1.5
Relative standard deviation: NMT 0.73%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of oxymetholone (C21H32O3) in the portion of Oxymetholone taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Oxymetholone RS in the Standard solution (mg/mL)
CU = concentration of Oxymetholone in the Sample solution (mg/mL)▲ (USP 1-May-2021)
Acceptance criteria: 97.0%–103.0% on the dried basis
Add the following:
4 IMPURITIES
Organic Impurities
[Note - Protect solutions containing oxymetholone from light.]
System suitability solution: 0.1 mg/mL of USP Oxymetholone RS and 0.02 mg/mL of USP Oxymetholone Related Compound B RS in toluene
Sensitivity solution: 0.001 mg/mL of USP Oxymetholone Related Compound B RS in toluene
Standard solution: 0.01 mg/mL of USP Oxymetholone RS in toluene
Sample solution: 1.0 mg/mL of Oxymetholone in toluene
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm × 30-m capillary; coated with a 0.25-μm film of phase G27
Temperatures
Injection port: 240°
Detector: 325°
Column: See Table 1.
Table 1
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
|---|---|---|---|
| 200 | 2 | 270 | 5 |
Carrier gas: Helium
Flow rate: 1.5 mL/min
Injection volume: 2 μL
Injection type: Split ratio, 5:1
[Note - The use of a deactivated inlet liner is recommended.]
System suitability
Samples: System suitability solution, Sensitivity solution, and Standard solution
[Note - See Table 2 for the relative retention times.]
Suitability requirements
Resolution: NLT 2.0 between oxymetholone and oxymetholone related compound B, System suitability solution
Tailing factor: NMT 1.2 for oxymetholone, Standard solution
Signal-to-noise ratio: NLT 10 for oxymetholone related compound B, Sensitivity solution
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Oxymetholone taken:
Result = (rU/Fi) × {1/[rT + Σ(rU/Fi)]} × 100
rU = peak response of each impurity from the Sample solution
Fi = relative response factor for each corresponding impurity (see Table 2)
rT = peak response of Oxymetholone from the Sample solution
Acceptance criteria: See Table 2. The reporting threshold is 0.1%.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Dimethylandrostadiolᵃ | 0.71 | 1.4 | 1.0 |
| Mestanoloneᵇ | 0.76 | 1.4 | 1.0 |
| Oxymetholone | 1.0 | – | – |
| Oxymetholone related compound B | 1.1 | 1.0 | 1.0 |
| 2-Formyl-3-methoxy androstanolᶜ | 1.2 | 1.1 | 1.0 |
| Any individual unspecified impurity | – | 1.0 | 0.5 |
| Total impurities | – | – | 2.0 |
a 3α,17α-Dimethyl-5α-androstan-3β,17β-diol.
b 17β-Hydroxy-17α-methyl-5α-androstan-3-one.
c 2-Formyl-3-methoxy-17α-methyl-5α-androsta-2-en-17β-ol.
5 SPECIFIC TESTS
Delete the following:
Melting Range or Temperature 〈741〉: 172°–180° (USP 1-May-2021)
Optical Rotation 〈781S〉, Procedures, Specific Rotation
Sample solution: 20 mg/mL in dioxane
Acceptance criteria: +34° to +38°
Loss on Drying 〈731〉
Analysis: Dry under vacuum over phosphorus pentoxide for 4 h.
Acceptance criteria: NMT 1.0%
Delete the following:
Completeness of Solution
Sample solution: 20 mg/mL in dioxane
Acceptance criteria: The solution is clear and free from undissolved solid. (USP 1-May-2021)
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers.
Change to read:
USP Reference Standards 〈11〉
USP Oxymetholone RS
USP Oxymetholone Related Compound B RS
17β-Hydroxy-1-hydroxymethylene-17α-methyl-5α-androstan-3-one.
C21H32O3 332.48 (USP 1-May-2021)


