Oxycodone Terephthalate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
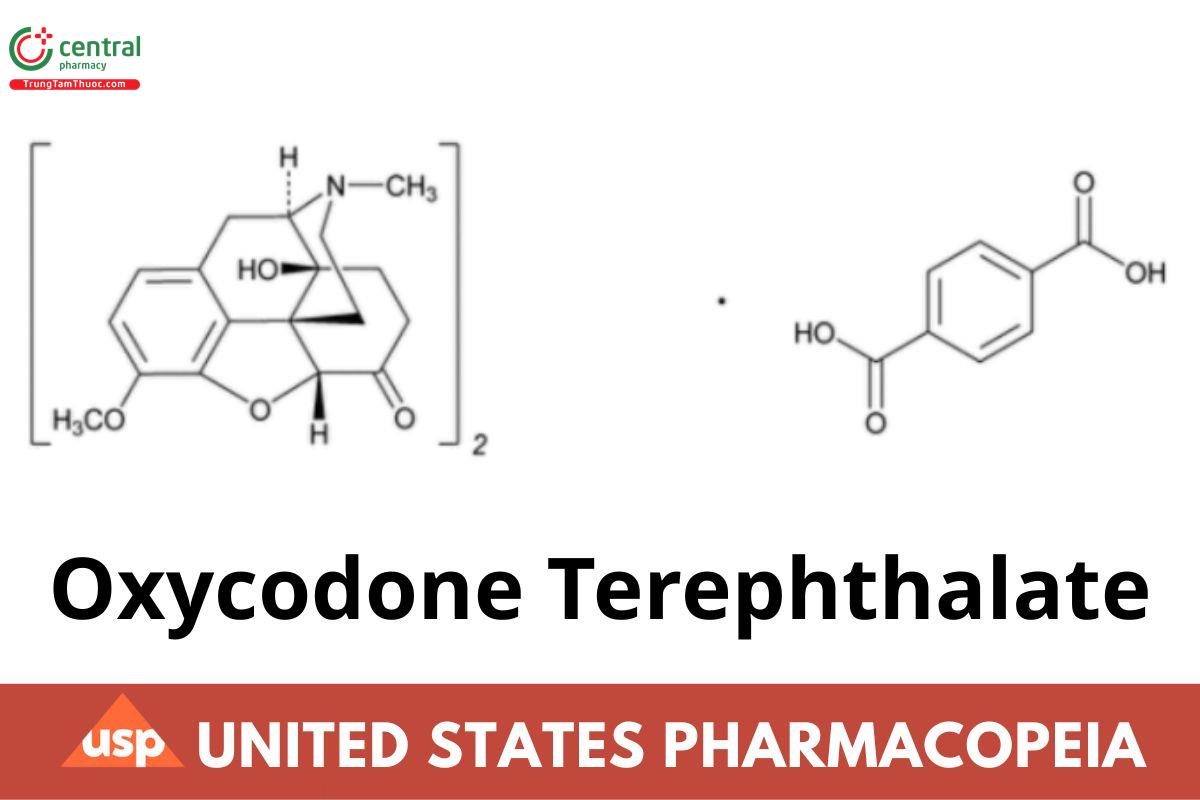
(C18H21NO4) · C8H6O4 796.86
Morphinan-6-one, 4,5-epoxy-14-hydroxy-3-methoxy-17-methyl-, 1,4-benzenedicarboxylate (2:1 salt), (5α); 4,5α-Epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one 1,4-benzenedicarboxylate (2:1 salt) CAS RN®: 64336-55-6.
1 DEFINITION
Oxycodone Terephthalate contains NLT 97.0% and NMT 103.0% of oxycodone terephthalate (C18H21NO4) · C8H6O4, calculated on the dried basis.
2 IDENTIFICATION
A. Melting Range or Temperature 〈741〉
Sample solution: Transfer 50 mL of the filtrate retained from the test for Content of Terephthalate Acid to a 125-mL conical flask. Render the solution alkaline with 6 N ammonium hydroxide. Allow the mixture to stand until a precipitate is formed. Filter, wash the precipitate with 50 mL of cold water, and dry for 2 h at 105°.
Acceptance criteria: The precipitate melts between 218° and 223°, but the range between the beginning and end of the melting does not exceed 2°.
Change to read:
B. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
Sample: Use a portion of the dried precipitate obtained in Identification test A.
Acceptance criteria: Meets the requirements
Change to read:
C. Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U (CN 1-May-2020)
Sample solution: 150 µg/mL in 0.1 N hydrochloric acid
Acceptance criteria: Exhibits a maxima at 280 nm
3 ASSAY
Procedure
Mobile phase: To 2.2 g of sodium 1-octanesulfonate in 740 mL of water add 260 mL of methanol, 10 mL of glacial acetic acid, and 0.1 mL of triethylamine. Mix, and adjust with 5 N sodium hydroxide to a pH of 6.5 ± 0.1. Pass through a filter of 0.5-µm or finer pore size. Diluent: 0.1 N hydrochloric acid
Internal standard solution: 0.1 mg/mL of ethylparaben prepared by dissolving in 2% of the ask volume of methanol and diluting with Diluent to volume
Standard stock solution: 0.75 mg/mL of USP Oxycodone RS in Diluent
Standard solution: 0.11 mg/mL of USP Oxycodone RS prepared as follows. Transfer 15.0 mL of Standard stock solution to a 100-mL volumetric flask, add 20.0 mL of Internal standard solution, and dilute with Diluent to volume.
Sample stock solution: 0.71 mg/mL of Oxycodone Terephthalate in Diluent. Filter, discarding the first 5 mL.
Sample solution: 0.14 mg/mL of Oxycodone Terephthalate prepared as follows. Transfer 10.0 mL of the Sample stock solution to a 50-mL volumetric flask, add 10.0 mL of Internal standard solution, and dilute with Diluent to volume.
3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 3.9-mm × 15-cm; packing L1
Column temperature: 50 ± 1.0°
Flow rate: 1 mL/min
Run time: Twice the retention time of the main oxycodone peak
Injection size: 30 µL
3.2 System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 6 between oxycodone and ethylparaben
Column efficiency: NLT 1800 theoretical plates
Relative standard deviation: NMT 2.0%
3.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of oxycodone terephthalate (C18H21NO4) · C8H6O4 in the portion of Oxycodone Terephthalate taken:
Result = (RU /RS) × (CS /CU) × (Mr1 /Mr2) × 100
RU = peak response ratio of oxycodone to ethylparaben from the Sample solution
RS = peak response ratio of oxycodone to ethylparaben from the Standard solution
CS = concentration of USP Oxycodone RS in the Standard solution (mg/mL)
CU = concentration of oxycodone in the Sample solution (mg/mL)
Mr1 = one-half of the molecular weight of oxycodone terephthalate, 398.43
Mr2 = molecular weight of oxycodone, 315.37
Acceptance criteria: 97.0%–103.0% on the dried basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 1%
4.1 Organic Impurities
Solution A: 2.2 g of sodium 1-octanesulfonate in 850 mL of water. Add 150 mL of methanol, 20 mL of glacial acetic acid, and 1.0 mL of triethylamine. Pass through a filter of 0.5-µm or finer pore size.
Solution B: 2.2 g of sodium 1-octanesulfonate in 500 mL of water. Add 500 mL of methanol, 20 mL of glacial acetic acid, and 1.0 mL of triethylamine. Pass through a filter of 0.5-µm or finer pore size.
Mobile phase: See Table 1.
Table 1
Time (min) | Solution A (%) | Solution B (%) |
0 | 90 | 10 |
30 | 80 | 20 |
50 | 0 | 100 |
55 | 0 | 100 |
Diluent: 0.1 N hydrochloric acid
Standard stock solution: 0.9 mg/mL of USP Oxycodone RS in Diluent
Standard solution: 0.09 mg/mL of USP Oxycodone RS from the Standard stock solution, prepared by adding to 20% of the ask volume of methanol, and diluting with Diluent to volume
System suitability stock solution: 0.05 mg/mL of 4-hydroxybenzoic acid isopropyl ester in methanol
System suitability solution: 0.01 mg/mL of 4-hydroxybenzoic acid isopropyl ester and 0.09 mg/mL of USP Oxycodone RS in Diluent from the System suitability stock solution and Standard stock solution, respectively
Sample solution: 11 mg/mL of Oxycodone Terephthalate in methanol prepared as follows. Transfer the required amount of sample to a suitable volumetric flask. Add 80% of the ask volume of methanol, and shake by mechanical means for about 20 min to dissolve. Dilute with methanol to volume.
4.2 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 3.9-mm × 15-cm; packing L1
Column temperature: 45 ± 1°
Flow rate: 1.5 mL/min
Injection size: 25 µL
4.3 System suitability
Samples: Standard solution and System suitability solution
Suitability requirements
Resolution: NLT 8 between the oxycodone and 4-hydroxybenzoic acid isopropyl ester peaks, System suitability solution Relative standard deviation: NMT 5.0%, Standard solution
4.4 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of the sample taken:
Result = (rU /rS) × (CS /CU) × (Mr1 /Mr2) × 100
rU = peak area of an individual impurity from the Sample solution
rS = peak area of oxycodone from the Standard solution
CS = concentration of USP Oxycodone RS in the Standard solution (mg/mL)
CU = concentration of Oxycodone Terephthalate in the Sample solution (mg/mL)
Mr1 = one-half of the molecular weight of oxycodone terephthalate, 398.43
Mr2 = molecular weight of oxycodone, 315.37
[Note—If any impurity is found having a retention time of about 2 in relation to that of the oxycodone peak, divide its apparent percentage by 4.8.]
Acceptance criteria
Individual impurities: NMT 1.0%
Total impurities: NMT 2.0%
5 SPECIFIC TESTS
Content of Terephthalic Acid
Sample solution: Transfer 1 g into a 50-mL beaker. Add 25 mL of 0.2 N hydrochloric acid, and heat to boiling with continuous stirring. Cover the beaker with a watch glass, and allow to cool to room temperature. Pass the suspension through a tared, medium-porosity filtering crucible. Transfer any material remaining in the beaker to the crucible with the aid of small portions of cold 0.2 N hydrochloric acid. Wash the material in the crucible with several portions of cold 0.2 N hydrochloric acid. [Note—Reserve the combined filtrates for use in Identification test A.]
Analysis: Dry the material in the crucible at 105° for 1 h, allow to cool, and reweigh. The material in the crucible is terephthalic acid. Determine the weight of terephthalic acid, and calculate the percentage of terephthalic acid.
Acceptance criteria: Between 20.2% and 21.5% of terephthalic acid (C8H6O4) in Oxycodone Terephthalate on the dried basis
Loss on Drying 〈731〉: Dry a sample at 105° for 4 h: it loses NMT 1.5% of its weight.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
USP Reference Standards 〈11〉
USP Oxycodone RS


