Oxaliplatin
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
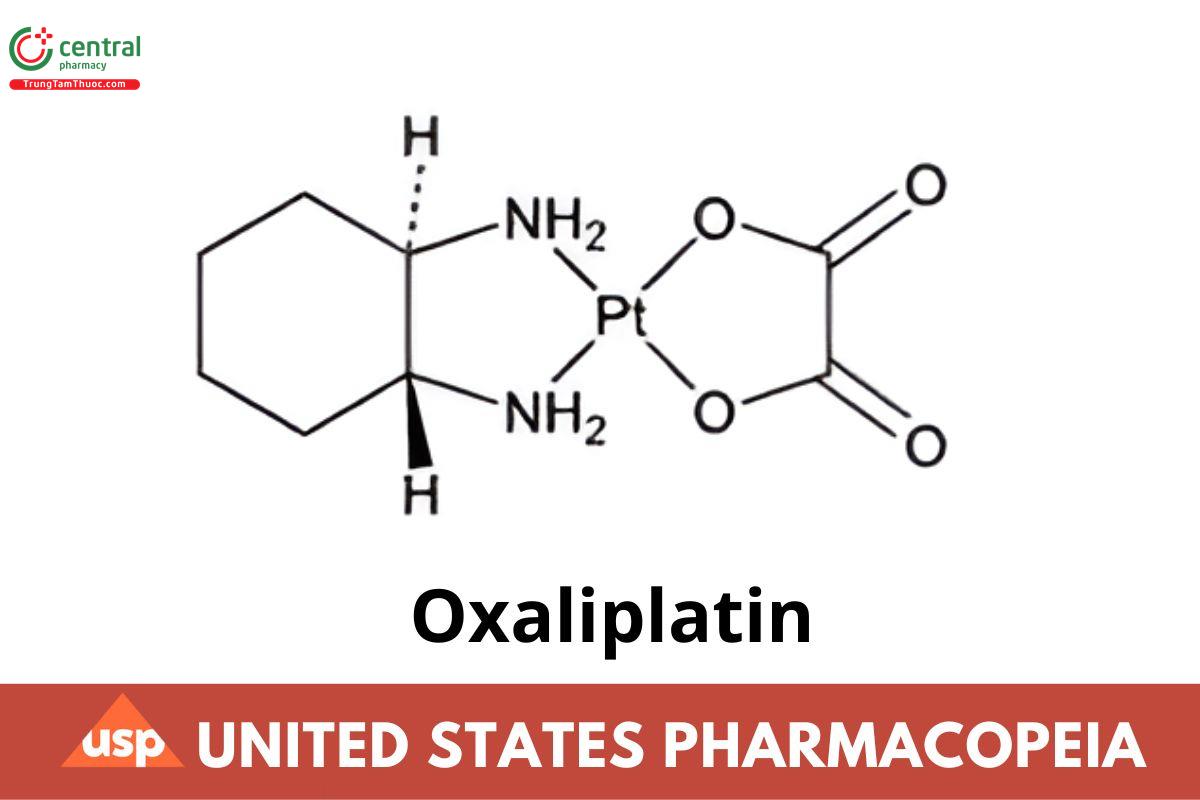
C8H14N2O4Pt 397.29
[SP-4-2-(1R-trans)]-(1,2-Cyclohexanediamine-N,N′)[ethanedioato(2-)-O,O′]platinum;
cis-[(1R,2R)-1,2-Cyclohexanediamine-N,N′][oxalato(2-)- O,O′]platinum CAS RN ®: 61825-94-3; UNII: 04ZR38536J.
1 DEFINITION
Oxaliplatin contains NLT 98.0% and NMT 102.0% of oxaliplatin (C8H14N2O4Pt), calculated on the dried basis. [Caution - Great care should be taken in handling Oxaliplatin, because it is a potentially cytotoxic agent.]
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
[Note - Use vigorous shaking and very brief sonication to dissolve the substance to be examined. Inject the Sample solution within 20 min of preparation. Polypropylene HPLC autosampler vials should be used.]
Buffer: Weigh 2.72 g of monobasic potassium phosphate (anhydrous) and 1.80 g of 1-pentanesulfonic acid sodium salt into a suitable container. Add 2000 mL of water, and mix well to completely dissolve all solids. Transfer 0.5 mL of triethylamine to the buffer solution, and mix thoroughly. Adjust the solution by dropwise addition of phosphoric acid to a pH of 4.30 ± 0.05.
Mobile phase: Methanol and Buffer (3:17)
Oxaliplatin standard stock solution: 0.5 mg/mL of USP Oxaliplatin RS in water
Oxaliplatin related compound B standard stock solution: Transfer USP Oxaliplatin Related Compound B RS to a suitable volumetric flask, add 25% of the final volume of methanol, and sonicate for approximately 2 min to disperse the solids. Add approximately 65% of the final volume of 0.001 M nitric acid, and sonicate for an additional 30 min to dissolve the solids. Allow to cool if necessary. Dilute with 0.001 M nitric acid to volume, and mix to obtain a solution having a known concentration of 0.125 mg/mL. [Note - USP Oxaliplatin Related Compound B RS is converted to (SP-4-2)-diaqua[(1R,2R)-cyclohexane-1,2-diamine-N,N′]platinum during preparation of this solution.]
Oxaliplatin related compound C standard stock solution: 0.1 mg/mL of USP Oxaliplatin Related Compound C RS in water
System suitability solution: 2 mg/mL of Oxaliplatin in 0.005 M sodium hydroxide. Allow this solution to stand at room temperature for at least 5 days. Transfer 10 mL of this solution, 10 mL of Oxaliplatin related compound B standard stock solution, and 5 mL of Oxaliplatin related compound C standard stock solution into a 100-mL volumetric flask, and dilute with water to volume. [Note—The preparation of the System suitability solution forms diaquodiaminocyclohexaneplatinum dimer.]
Standard solution: 0.1 mg/mL of USP Oxaliplatin RS in water from Oxaliplatin standard stock solution
Sample solution: 0.1 mg/mL of Oxaliplatin in water
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 210 nm
Column: 4.6-mm × 25-cm; 5-μm packing L1
Flow rate: 1 mL/min
Injection volume: 50 μL
System suitability
Samples: System suitability solution and Standard solution
[Note - The relative retention times, measured with respect to oxaliplatin, of oxaliplatin related compound C, oxaliplatin related compound B, and diaquodiaminocyclohexaneplatinum dimer are 0.8, 2.7, and 6, respectively.]
Suitability requirements
Resolution: NLT 2.0 between oxaliplatin and oxaliplatin related compound C, System suitability solution
Tailing factor: Between 0.8 and 2.0 for oxaliplatin, System suitability solution
Relative standard deviation: NMT 2.0% for oxaliplatin, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of oxaliplatin (C8H14N2O4Pt) in the portion of Oxaliplatin taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Oxaliplatin RS in the Standard solution (mg/mL)
CU = concentration of Oxaliplatin in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the dried basis
4 IMPURITIES
4.1 Limit of Silver
Sample stock solution: Dissolve 100 mg of Oxaliplatin, weighed, in 50 mL of water to obtain a solution having a concentration of 2 mg/mL.
Sample solution: 1 mg/mL of Oxaliplatin in 0.5 M nitric acid from the Sample stock solution
Standard stock solution: Dilute a commercially available silver nitrate atomic absorption standard solution containing 1000 ppm of silver in 0.5 M nitric acid quantitatively, and stepwise if necessary, with 0.5 M nitric acid to obtain a 10-ppb solution.
Standard solution 1: Mix 20 μL of the Sample stock solution and 8 μL of the Standard stock solution, and dilute with 0.5 M nitric acid to 40 μL.
Standard solution 2: Mix 20 μL of the Sample stock solution and 16 μL of the Standard stock solution, and dilute with 0.5 M nitric acid to 40 μL.
Instrumental conditions
(See Atomic Absorption Spectroscopy 〈852〉.)
Mode: Atomic absorption spectrophotometer equipped with a silver hollow-cathode lamp and graphite furnace
Analytical wavelength: Silver emission line of 328.1 nm
Blank: 0.5 M nitric acid
Analysis
Samples: Sample solution, Standard solution 1, and Standard solution 2
Plot the absorbances of the Sample solution, Standard solution 1, and Standard solution 2 versus their concentrations, in ppb, of silver, and draw the straight line best fitting the three plotted points. The intercept on the x-axis of the extended regression line indicates the silver concentration in the Sample solution.
Calculate the concentration of silver, in ppm, in the portion of Oxaliplatin taken:
Result = (C/W) × 100
C = absolute value of the intercept, in ppb of silver, on the x-axis
W = weight of Oxaliplatin taken for the preparation of the Sample stock solution (mg)
Acceptance criteria: NMT 5 ppm
4.2 Content of Platinum
Sample: Ignite an empty porcelain crucible fitted with a lid in a furnace at 800° for 30 min. Cool in a desiccator, and weigh. Add 200 mg of the Oxaliplatin, weighed, to the crucible, and ignite in a furnace by stepwise increments as follows. Introduce into the furnace, and increase the temperature to 200° within 15 min, then to 400° within 15 min, then to 600° within 15 min, then finally to 800° within 15 min. Allow to remain in the furnace at 800° for 30 min. Remove, cool in a desiccator, and reweigh.
Calculate the percentage of platinum in the portion of Oxaliplatin taken:
Result = (W2/W1) × 100
W2 = weight of residue after ignition (mg)
W1 = weight of oxaliplatin before ignition (mg)
Acceptance criteria: 48.1%–50.1% of the oxaliplatin taken, on the dried basis
Change to read:
4.3 Organic Impurities, Procedure 1: Limit of Oxalic Acid
[Note - Use vigorous shaking and very brief sonication to dissolve the substance to be examined. Inject the Sample solution within 20 min of preparation. Polypropylene HPLC autosampler vials should be used.]
Buffer: Add 1.36 g of potassium dihydrogen phosphate to 10 mL of 10% tetrabutylammonium hydroxide in water, and dilute with water to 1000 mL. Adjust with phosphoric acid to a pH of 6.0.
Mobile phase: Acetonitrile and Buffer (1:4)
Standard stock solution: 0.06 mg/mL of USP Oxaliplatin Related Compound A RS in water
Standard solution: 15 μg/mL of USP Oxaliplatin Related Compound A RS in water from the Standard stock solution
System suitability solution: 0.05 mg/mL of sodium nitrate in water. Transfer 2 mL of this solution and 25 mL of the Standard stock solution to a 100-mL volumetric flask, and dilute with water to volume.
Sensitivity solution: 1.5 μg/mL of USP Oxaliplatin Related Compound A RS in water from the Standard solution
Sample solution: 2 mg/mL of Oxaliplatin in water
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 205 nm
Column: 4.6-mm × 25-cm; 5-μm packing L1
Column temperature: 40°
Flow rate: 2 mL/min
Injection volume: 20 μL
System suitability
Samples: Standard solution, System suitability solution, and Sensitivity solution
[Note - The elution order is sodium nitrate, followed by oxalic acid.]
Suitability requirements
Resolution: NLT 2.0 between oxalic acid and sodium nitrate, System suitability solution
Relative standard deviation: NMT 3.0% for oxalic acid, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of oxalic acid in the portion of Oxaliplatin taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
rU = peak response of oxalic acid from the Sample solution
rS = peak response of oxalic acid from the Standard solution
CS = concentration of USP Oxaliplatin Related Compound A RS in the Standard solution (mg/mL)
CU = concentration of Oxaliplatin in the Sample solution (mg/mL)
Mr1 = molecular weight of anhydrous oxalic acid, 90.03
Mr2 = molecular weight of USP Oxaliplatin Related Compound A RS, 126.07
Acceptance criteria: NMT 0.1%
4.4 Organic Impurities, Procedure 2
Limit of (SP-4-2-)-Diaqua[(1R,2R)-cyclohexane-1,2-diamine-N,N′]platinum, Oxaliplatin Related Compound C, and Unspecified Impurities
[Note - Use vigorous shaking and very brief sonication to dissolve the substance to be examined. Inject the Sample solution within 20 min of preparation. Polypropylene HPLC autosampler vials should be used.]
Mobile phase, Oxaliplatin standard stock solution, Oxaliplatin related compound B standard stock solution, Oxaliplatin related compound C standard stock solution, System suitability solution, and Chromatographic system: Proceed as directed in the Assay.
Standard solution: 0.01 mg/mL of oxaliplatin, 0.01 mg/mL of oxaliplatin related compound B, and 0.004 mg/mL of oxaliplatin related compound C in water from Oxaliplatin standard stock solution,Oxaliplatin related compound B standard stock solution, and Oxaliplatin related compound C standard stock solution, respectively
Sample solution: 2 mg/mL of Oxaliplatin in water
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 2.0 between oxaliplatin and oxaliplatin related compound C, System suitability solution
Tailing factor: Between 0.8 and 2.0 for oxaliplatin, System suitability solution
Relative standard deviation: NMT 3.0% for oxaliplatin, oxaliplatin related compound B, and oxaliplatin related compound C, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of (SP-4-2)-diaqua[(1R,2R)-cyclohexane-1,2-diamine-N,N′]platinum in the portion of Oxaliplatin taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
rU = peak response of (SP-4-2)-diaqua[(1R,2R)-cyclohexane-1,2-diamine-N,N′]platinum from the Sample solution
rS = peak response of (SP-4-2)-diaqua[(1R,2R)-cyclohexane-1,2-diamine-N,N′]platinum from the Standard solution
CS = concentration of USP Oxaliplatin Related Compound B RS in the Standard solution (mg/mL)
CU = concentration of Oxaliplatin in the Sample solution (mg/mL)
Mr1 = molecular weight of (SP-4-2)-diaqua[(1R,2R)-cyclohexane-1,2-diamine-N,N′]platinum, 345.30
Mr2 = molecular weight of USP Oxaliplatin Related Compound B RS, 433.28
[Note - USP Oxaliplatin Related Compound B RS is converted to (SP-4-2)-diaqua[(1R,2R)-cyclohexane-1,2-diamine-N,N′]platinum in solution preparation.]
Calculate the percentage of oxaliplatin related compound C in the portion of Oxaliplatin taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of oxaliplatin related compound C from the Sample solution
rS = peak response of oxaliplatin related compound C from the Standard solution
CS = concentration of USP Oxaliplatin Related Compound C RS in the Standard solution (mg/mL)
CU = concentration of Oxaliplatin in the Sample solution (mg/mL)
Calculate the percentage of diaquodiaminocyclo hexaneplatinum dimer in the portion of Oxaliplatin taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2)× (1/F) × 100
rU = peak response of diaquodiaminocyclo hexaneplatinum dimer from the Sample solution
rS = peak response of oxaliplatin related compound B from the Standard solution
CS = concentration of USP Oxaliplatin Related Compound B RS in the Standard solution (mg/mL)
CU = concentration of Oxaliplatin in the Sample solution (mg/mL)
Mr1 = molecular weight of (SP-4-2)-diaqua[(1R,2R)-cyclohexane-1,2-diamine-N,N′]platinum, 345.30
Mr2 = molecular weight of USP Oxaliplatin Related Compound B RS, 433.28
F = relative response factor for diaquodiami nocyclohexaneplatinum dimer, measured with respect to USP Oxaliplatin Related Compound B RS, 2.5
Calculate the percentage of any other unspecified impurity in the portion of Oxaliplatin taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of any other unspecified impurity from the Sample solution
rS = peak response of oxaliplatin from the Standard solution
CS = concentration of oxaliplatin in the Standard solution (mg/mL)
CU = concentration of Oxaliplatin in the Sample solution (mg/mL)
Acceptance criteria: See Table 1.
Table 1
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Oxaliplatin related compound C | 0.8 | – | 0.1 |
| Oxaliplatin | 1.0 | – | – |
| (SP-4-2)-Diaqua[(1R,2R)-cyclohexane-1,2-diamine-N,N′]platinum | 2.7 | – | 0.1 |
| Diaquadiaminocyclohexaneplatinum dimer | 6 | 2.5 | 0.1 |
| Any individual unspecified impurity | – | – | 0.10 |
| Total impuritiesᵃ | – | – | 0.30 |
a Total impurities include oxalic acid (from Procedure 1) and all impurities from Procedure 2.
4.5 Organic Impurities, Procedure 3
Limit of Oxaliplatin Related Compound D
[Note - Use vigorous shaking and very brief sonication to dissolve the substance to be examined. Inject the Sample solution within 20 min of preparation. Polypropylene HPLC autosampler vials should be used.]
Mobile phase: Methanol and Ethanol (7:3)
Oxaliplatin related compound D standard stock solution: 0.05 mg/mL of USP Oxaliplatin Related Compound D RS in methanol
Oxaliplatin related compound D standard solution: 15 μg/mL of USP Oxaliplatin Related Compound D RS in methanol from Oxaliplatin related compound D standard stock solution
Oxaliplatin standard stock solution: 0.75 mg/mL of USP Oxaliplatin RS in methanol
Oxaliplatin standard solution: 37.5 μg/mL of USP Oxaliplatin RS in methanol from Oxaliplatin standard stock solution
Oxaliplatin blank solution: Transfer 40 mL of Oxaliplatin standard stock solution to a 50-mL volumetric flask, and dilute with methanol to volume.
Standard solutions: Transfer 40 mL of Oxaliplatin standard stock solution to separate 50-mL volumetric flasks. Add 1.0, 3.0, and 5.0 mL of Oxaliplatin related compound D standard solution to each flask, and dilute with methanol to volume. The concentration of oxaliplatin in these solutions is 0.6 mg/mL. The concentrations of oxaliplatin related compound D in these solutions are 0.3, 0.9, and 1.5 μg/mL, respectively.
System suitability solution: Transfer 5.0 mL of Oxaliplatin standard solution and 4.0 mL of Oxaliplatin related compound D standard stock solution to a 50-mL volumetric flask, and dilute with methanol to volume.
Sample solution: Transfer 30 mg of Oxaliplatin into a 50-mL volumetric flask, and dilute with methanol to volume.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 25-cm; 5-μm packing L70
Column temperature: 40°
Flow rate: 0.3 mL/min
Injection volume: 20 μL
Run time: 30 min
System suitability
Samples: 0.9-μg/mL Standard solution and System suitability solution
Suitability requirements
Resolution: NLT 1.5 between oxaliplatin and oxaliplatin related compound D, System suitability solution
Relative standard deviation: NMT 3.0% for the peak height ratio of oxaliplatin related compound D to the sum of oxaliplatin and oxaliplatin related compound D; 0.9-μg/mL Standard solution
Analysis
Samples: Standard solutions and Sample solution
Subtract the oxaliplatin related compound D peak height obtained in the Oxaliplatin blank solution from the oxaliplatin related compound D peak height obtained in the Standard solutions. [Note - USP Oxaliplatin RS may contain a small amount of oxaliplatin related compound D.] Plot a calibration curve for the Standard solutions with the peak height ratios of oxaliplatin related compound D to the sum of oxaliplatin and oxaliplatin related compound D on the y-axis and the concentration ratios of oxaliplatin related compound D, in μg/mL, to the sum of oxaliplatin and oxaliplatin related compound D concentrations, in mg/mL, on the x-axis. Read the concentration ratio of oxaliplatin related compound D, in μg/mL, to the sum of oxaliplatin and oxaliplatin related compound D, in mg/mL, in the Sample solution from the calibration curve.
Calculate the percentage of oxaliplatin related compound D in the portion of Oxaliplatin taken:
Result = R/10
R = concentration ratio of oxaliplatin related compound D, in μg/mL, to the sum of oxaliplatin and oxaliplatin related compound D, in mg/mL, in the Sample solution from the calibration curve
Acceptance criteria: NMT 0.1%
5 SPECIFIC TESTS
Acidity
Sample solution: Dissolve 100 mg in 50 mL of carbon dioxide-free water, and add 0.5 mL of phenolphthalein TS.
Acceptance criteria: The solution is colorless, and NMT 0.6 mL of 0.01 M sodium hydroxide is required to change the color to pink.
Bacterial Endotoxins Test 〈85〉: NMT 1.0 USP Endotoxin Unit/mg of oxaliplatin
Loss on Drying 〈731〉
Analysis: Dry 1 g at 100°–105° for 2 h.
Acceptance criteria: NMT 0.5%
Microbial Enumeration Tests 〈61〉 and Tests for Specified Microorganisms 〈62〉: The total aerobic microbial count does not exceed 20 cfu/g, and the total combined molds and yeast count does not exceed 5 cfu/g.
Optical Rotation, Specific Rotation 〈781S〉
Sample solution: 5 mg/mL in water
Acceptance criteria: Between +74.5° and +78.0°, measured at 20°
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers, protected from light. Store at room temperature.
USP Reference Standards 〈11〉
USP Oxaliplatin RS
USP Oxaliplatin Related Compound A RS
Oxalic acid dihydrate.
C2H2O4 · 2H O 126.07
USP Oxaliplatin Related Compound B RS
[SP-4-2-(1R-trans)]-(1,2-Cyclohexanediamine-N,N′) dinitratoplatinum(II).
C6H14N4O6Pt 433.28
USP Oxaliplatin Related Compound C RS
[1R-trans-(1,2-Cyclohexanediamine-N,N′)]-trans-dihydroxido-[oxalato(2-)-O,O′]platinum(IV).
C8H16N2O6Pt 431.30
USP Oxaliplatin Related Compound D RS
cis-[(1S,2S)-1,2-Cyclohexanediamine-N,N′][oxalato(2-)-O,O′]platinum.
C8H14N2O4Pt 397.29


