Oseltamivir Phosphate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
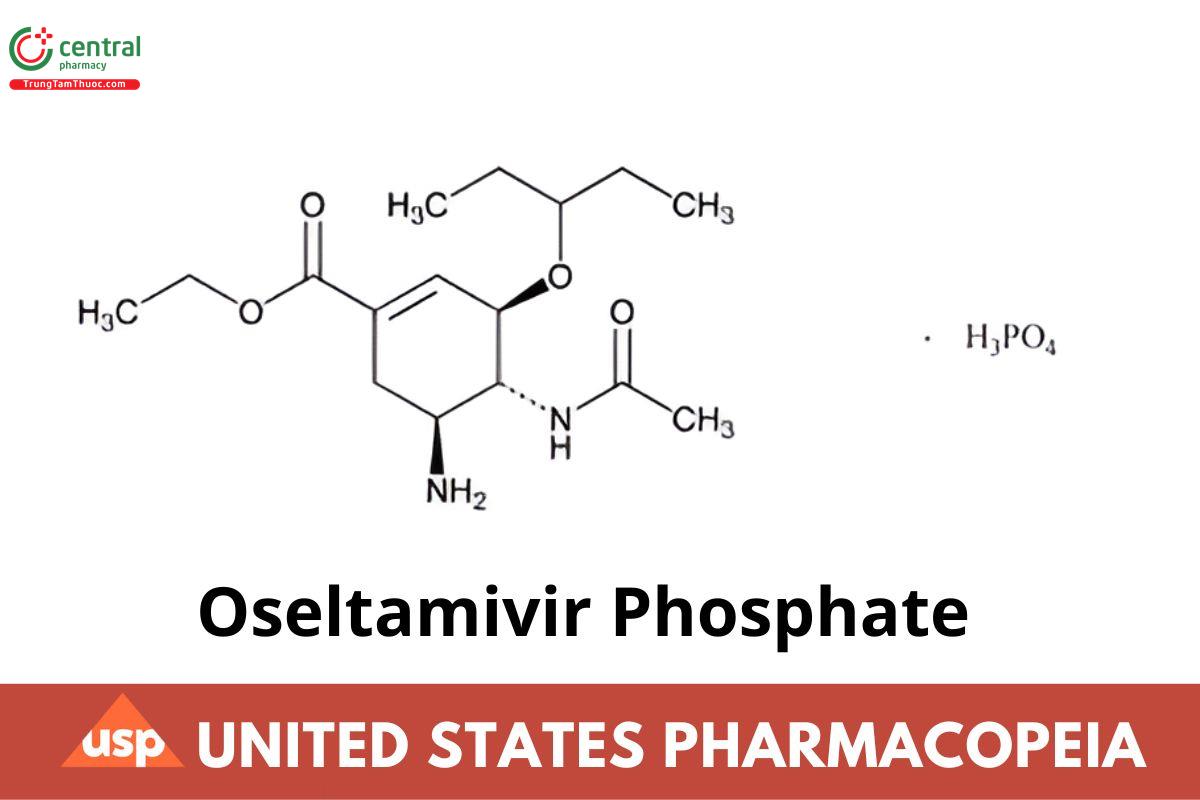
C16H28N2O4 · H3PO4 410.40
[3R-(3α,4β,5α)]-Ethyl 4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate phosphate (1:1);
Ethyl (3R,4R,5S)-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate, phosphate (1:1) CAS RN ®: 204255-11-8; UNII: 4A3O49NGEZ.
1 DEFINITION
Oseltamivir Phosphate contains NLT 98.0% and NMT 101.5% of C16H28N2O4 · H3PO4 , calculated on the anhydrous basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Solution A: Dissolve 6.8 g of potassium dihydrogen phosphate in 980 mL of water. Adjust with 1 M potassium hydroxide solution to a pH of 6.0, and dilute with water to 1 L.
Mobile phase: Methanol, acetonitrile, and Solution A (245:135:620)
Diluent: Methanol, acetonitrile, and water (245:135:620)
Standard solution: 1 mg/mL of USP Oseltamivir Phosphate RS in Diluent
Sample solution: 1 mg/mL of Oseltamivir Phosphate in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 207 nm
Column: 4.6-mm × 25-cm; packing L7
Column temperature: 50°
Flow rate: 1.2 mL/min
Injection size: 15 μL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of C16H28N2O4 · H3PO4 in the portion of Oseltamivir Phosphate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Oseltamivir Phosphate RS in the Standard solution (mg/mL)
CU = concentration of Oseltamivir Phosphate in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–101.5% on the anhydrous basis
4 IMPURITIES
Change to read:
Organic Impurities
4.1 Procedure 1
Solution A, Mobile phase, Diluent, Standard solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Oseltamivir Phosphate taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU = peak response of each individual impurity from the Sample solution
rS = peak response of oseltamivir phosphate from the Standard solution
CS = concentration of USP Oseltamivir Phosphate RS in the Standard solution (mg/mL)
CU = concentration of Oseltamivir Phosphate in the Sample solution (mg/mL)
F = relative response factor from Impurity Table 1
Acceptance criteria
Individual impurities: See Impurity Table 1.
Total impurities: NMT 0.7%
Impurity Table 1
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Oseltamivir acidᵃ | 0.17 | 1.4 | 0.3 |
| Oseltamivir phenolᵇ | 0.51 | 2.7 | 0.1 |
| Oseltamivir phosphate | 1.00 | 1.0 | – |
| Unspecified impurity | – | 1.0 | 0.1 |
| Total unspecified impurity | – | – | 0.4 |
a(3R,4R,5S)-4-Acetylamino-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid.
b 4-Acetylamino-3-hydroxybenzoic acid ethyl ester.
4.2 Procedure 2: Oseltamivir Related Compound A
Buffer: 1.54 g/L of ammonium acetate in water
Mobile phase: Acetonitrile, water, and Buffer (3:6:1)
Stock solution A: 50 μg/mL of USP Oseltamivir Related Compound A RS, prepared as follows: Dissolve in alcohol, using 5% of final volume, and dilute with water to volume.
Solution A: 1 μg/mL of USP Oseltamivir Related Compound A RS in water from Stock solution A
Standard solution: 10 mg/mL of USP Oseltamivir Phosphate RS in Solution A
Sample solution: 10 mg/mL of Oseltamivir Phosphate in water
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detectors: UV 210 nm and mass spectrometer
Column: 3.0-mm × 5-cm; 5-μm packing L1
Flow rate: 1.5 mL/min
Injection size: 1 μL
Temperature: 40°
Use electrospray (+) ionization, a selected ion monitoring mode with m/z of 356.2 (protonated oseltamivir related compound A). Adjust the dwell time, fragmentation voltage, drying gas temperature, drying gas flow, nebulizer pressure, and capillary voltage as appropriate for an optimal response. [Note - A postcolumn flow splitter with a split ratio of about 3:1 is used.]
System suitability
Sample: Standard solution
[Note - The relative retention time for oseltamivir related compound A versus oseltamivir is about 2.6.]
Suitability requirements
Resolution: The oseltamivir related compound A peak (detected by MD-SIM mode) and the oseltamivir peak (detected by UV) are baseline resolved.
[Note - The resolution of the two components minimizes background noise and ion suppression effects for the trace of oseltamivir related compound A by the oseltamivir matrix.]
Relative standard deviation: NMT 15.0%, oseltamivir related compound A peak
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of oseltamivir related compound A in the portion of Oseltamivir Phosphate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of oseltamivir related compound A from the Sample solution
rS = peak response of oseltamivir related compound A from the Standard solution
CS = concentration of USP Oseltamivir Related Compound A RS in the Standard solution (mg/mL)
CU = concentration of oseltamivir phosphate in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.01%
4.3 Procedure 3: Limit Of Tributyl Phosphine Oxide
Blank: Transfer 1.0 mL of suitable derivatizing reagent1 to a vial. Close the vial, shake, and heat for 20 min at 60°. Centrifuge the pyridinium salt precipitate.
Standard stock solution 1: 21 mg/mL of USP Tributyl Phosphine Oxide RS in pyridine
Standard stock solution 2: 21 mg/mL of USP Oseltamivir Phosphate RS in suitable derivatizing reagent. Close the vial, mix, and heat for 20 min at 60°. Centrifuge the pyridinium salt precipitate.
Standard solution: 21 μg/mL each of USP Tributyl Phosphine Oxide RS and USP Oseltamivir Phosphate RS in pyridine from Standard stock solution 1 and Standard stock solution 2, respectively
Sample solution: Transfer 15 mg of Oseltamivir Phosphate to a vial. Add 1.0 mL of suitable derivatizing reagent. Close the vial, mix, and heat for 20 min to 60°. Centrifuge the pyridinium salt precipitate.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm × 30-m capillary column coated with a 0.25-μm phase G1
Split ratio: 1:50
Split flow: 64 mL/min
Injection size: 1 μL
Temperature
Detector: 260°
Injection port: 260°
Column: See the temperature program table below.
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
|---|---|---|---|
| 180 | 0 | 180 | 2 |
| 180 | 8 | 250 | 10 |
Linear velocity: 27 cm/s
Carrier gas: Helium
System suitability
Sample: Standard solution
[Note - The relative retention times for tributyl phosphine oxide and oseltamivir phosphate are about 0.54 and 1.00, respectively.]
Suitability requirements
Relative standard deviation: NMT 10.0% for the tributyl phosphine oxide and oseltamivir (ERR 1-Jun-2023) phosphate peaks
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of tributyl phosphine oxide in the portion of Oseltamivir Phosphate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of tributyl phosphine oxide from the Sample solution
rS = peak response of tributyl phosphine oxide from the Standard solution
CS = concentration of tributyl phosphine oxide in the Standard solution (mg/mL)
CU = concentration of Oseltamivir Phosphate in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.1%
5 SPECIFIC TESTS
Water Determination, Method I〈921〉: NMT 0.5%
Optical Rotation, Specific Rotation 〈781S〉
Sample solution: 10 mg/mL in water
Acceptance criteria: Between –30.7 and –32.6
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. Store at 25°, excursions permitted between 15° and 30°.
USP Reference Standards 〈11〉
USP Oseltamivir Phosphate RS
USP Oseltamivir Related Compound A RS
(3S,4R,5S)-Ethyl 4-acetamido-5-amino-2-azido-3-(pentan-3-yloxy)cyclohexanecarboxylate.
C16H29N5O4 355.43
USP Tributyl Phosphine Oxide RS C H OP 218.32
1 Tri-Sil Reagent (product number: 48999 0049001) may be obtained from Pierce: www.piercenet.com.


