Orlistat
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
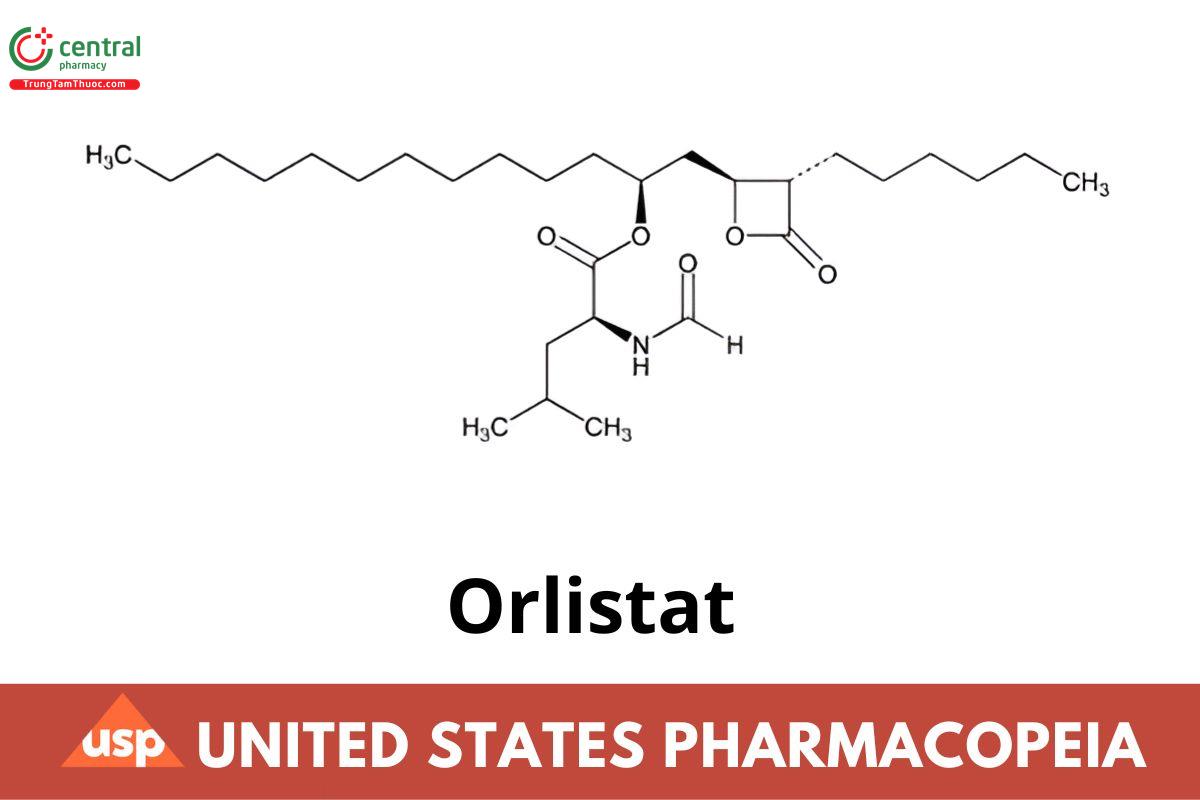
C29H53NO5 495.75 (IRA 1-Jan-2022)
l-Leucine, N-formyl-, 1-[(3-hexyl-4-oxo-2-oxetanyl)methyl]dodecyl ester, [2S-[2α(R*), 3β]]-;
N-Formyl-l-leucine, ester with (3S,4S)-3-hexyl-4-[(2S)-2-hydroxytridecyl]-2-oxetanone;
(S)-1-[(2S,3S)-3-Hexyl-4-oxooxetan-2-yl]tridecan-2-yl formyl-l-leucinate (IRA 1-Jan-2022) CAS RN®: 96829-58-2; UNII: 95M8R751W8.
1 DEFINITION
Orlistat contains NLT 98.0% and NMT 101.5% of orlistat (C H NO ), calculated on the anhydrous, solvent-free basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K or 197A (IRA 1-Jan-2022)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Change to read:
3.1 Procedure
3.2 [Note - Avoid the use of plastic flasks for the preparation or containment of any solution in this analysis.]
Mobile phase: Acetonitrile, phosphoric acid, and water (860: 0.05: 140)
Standard solution: 0.5 mg/mL of USP Orlistat RS in Mobile phase. Inject immediately after preparation or store at 5°.
Sample solution: 0.5 mg/mL of Orlistat in Mobile phase. Inject immediately after preparation or store at 5°.
3.3 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 195 nm (IRA 1-Jan-2022)
Column: 3.9-mm × 15-cm; 4-μm packing L1
Flow rate: 1.0 mL/min
Injection volume: 20 μL
Run time: NLT 5.5 times the retention time of orlistat (IRA 1-Jan-2022)
3.4 System suitability
Sample: Standard solution
3.5 Suitability requirements
Relative standard deviation: NMT 1.0% (IRA 1-Jan-2022)
3.6 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of orlistat (C29H53NO5) in the portion of Orlistat taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of orlistat from the Sample solution
rS = peak response of orlistat from the Standard solution
CS = concentration of USP Orlistat RS in the Standard solution (mg/mL)
CU = concentration of Orlistat in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–101.5% on the anhydrous, solvent-free basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
4.1 Limit of Orlistat Related Compound A
Standard solution: 0.1 mg/mL of USP Orlistat Related Compound A RS in acetone
Sample solution: 50 mg/mL of Orlistat in acetone
Chromatographic system
(See Chromatography 〈621〉, General Procedures, Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 10 μL
Developing solvent system: Toluene and ethyl acetate (4:1)
Detection solution: Transfer 2.5 g of phosphomolybdic acid and 1 g of ceric sulfate into a 100-mL volumetric flask, and dissolve in and dilute with methanol to volume.
Analysis
Samples: Standard solution and Sample solution
Remove the plate, and air-dry it thoroughly. Spray the dried plate with Detection solution, and place the plate in an oven at 120° for 30 min.
Acceptance criteria: Any secondary spot from the Sample solution corresponding to orlistat related compound A is not more intense than the corresponding spot from the Standard solution (0.2%).
4.2 Limit of Orlistat Related Compound B
Standard solution: 0.025 mg/mL of USP Orlistat Related Compound B RS in methylene chloride
Sample solution: 50 mg/mL of Orlistat in methylene chloride
Spiked sample solution: 50 mg/mL of Orlistat in Standard solution
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm × 30-m fused silica, coated with a 0.25-μm G27 stationary phase
Temperatures
Injector: 270°
Detector: 280°
Column: See Table 1.
Table 1
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
|---|---|---|---|
| 50 | 4 | 170 | – |
| 170 | 30 | 300 | 30 |
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 10.0%
Analysis
Samples: Standard solution, Sample solution, and Spiked sample solution
Calculate the percentage of orlistat related compound B in the portion of Orlistat taken:
Result = {rU/[rSP − rU × (CT/CU)]} × (CS/CU) × 100
rU = peak response of orlistat related compound B from the Sample solution
rSP = peak response of orlistat related compound B from the Spiked sample solution
CT = concentration of Orlistat in the Spiked sample solution (mg/mL)
CU = concentration of Orlistat in the Sample solution (mg/mL)
CS = concentration of USP Orlistat Related Compound B RS in the Standard solution (mg/mL)
Acceptance criteria: NMT 0.05%
Change to read:
4.3 Organic Impurities
[Note-Avoid the use of plastic flasks for the preparation or containment of any solution in this analysis.]
Mobile phase, Standard solution, and Sample solution: Prepare as directed in the Assay.
System suitability solution: 10 μg/mL of USP Orlistat RS and 0.1 μg/mL of USP Orlistat Related Compound C RS in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 195 nm
Column: 3.9-mm × 15-cm; 4-μm packing L1
Flow rate: 1.0 mL/min
Injection volume: 20 μL
Run time: NLT 5.5 times the retention time of orlistat (IRA 1-Jan-2022)
System suitability
Sample: System suitability solution
Suitability requirements
Relative standard deviation: NMT 5.0% (IRA 1-Jan-2022) for the orlistat peak
Signal-to-noise ratio: NLT 3 for orlistat related compound C
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity other than orlistat related compound A, orlistat related compound B, orlistat related compound
D, orlistat open ring amide, and orlistat related compound E in the portion of Orlistat taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU = peak response of each individual impurity from the Sample solution
rS = peak response of orlistat from the Standard solution
CS = concentration of USP Orlistat RS in the Standard solution (mg/mL)
CU = concentration of Orlistat in the Sample solution (mg/mL)
F = relative response factor (see Table 2)
Acceptance criteria: See Table 2.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Formylleucineᵃ | 0.10 | 4.0 | 0.2 |
| Orlistat related compound C | 0.13 | 33 | 0.05 |
| Orlistat open ring epimerᵇ | 0.44 | 1.0 | 0.2 |
| Orlistat related compound Dᶜ | 0.90 | – | see Table 3 |
| Orlistat open ring amideᶜ,ᵈ | 0.90 | – | see Table 3 |
| Orlistat | 1.00 | – | – |
| D-Leucine orlistatᵉ | 1.18 | 1.0 | 0.2 |
| Any individual unspecified impurity (IRA 1-Jan-2022) | – | 1.0 | 0.1 |
| Total impuritiesᶠ | – | – | 1.0 |
a N-Formyl-l-leucine.
b (2S,3R,5S)-5-[(Formyl-l-leucyl)oxy]-2-hexyl-3-hydroxyhexadecanoic acid. (IRA 1-Jan-2022)
c Coelutes in this LC system, determined using Limits of Orlistat Related Compound D and Orlistat Open Ring Amide.
d (7S,8S,10S)-8-Hydroxy-7-{[(R)-1-phenylethyl]carbamoyl}henicosan-10-yl formyl-l-leucinate. (IRA 1-Jan-2022)
e (S)-1-[(2S,3S)-3-Hexyl-4-oxooxetan-2-yl]tridecan-2-yl formyl-d-leucinate. (IRA 1-Jan-2022)
f Sum of results obtained in the tests for Limit of Orlistat Related Compound A, Limit of Orlistat Related Compound B, Organic Impurities, Limits of Orlistat Related Compound D and Orlistat Open Ring Amide, and Limit of Orlistat Related Compound E.
Change to read:
4.4 Limits of Orlistat Related Compound D and Orlistat Open Ring Amide
Mobile phase: Methanol and water (830:170)
System suitability solution: 4 mg/mL of USP Orlistat RS and 2.5 μg/mL of orlistat related compound D prepared by dissolving a suitable amount of USP Orlistat RS and diluting USP Orlistat Related Compound D Solution RS in acetonitrile (IRA 1-Jan-2022)
Standard solution: 5.0 mg/mL of USP Orlistat RS in acetonitrile
Sample solution: 5.0 mg/mL of Orlistat in acetonitrile
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 205 nm
Column: 4.0-mm × 25-cm; 5-μm packing L7
Flow rate: 0.6 mL/min
Injection volume: 20 μL
Run time: NLT 1.4 times the retention time of orlistat (IRA 1-Jan-2022)
System suitability
Sample: System suitability solution
Suitability requirements
Relative standard deviation: NMT 5.0% (IRA 1-Jan-2022) for the orlistat peak
Signal-to-noise ratio: NLT 3 for the orlistat related compound D peak
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of orlistat related compound D and orlistat open ring amide in the portion of Orlistat taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU = peak response of orlistat related compound D or orlistat open ring amide from the Sample solution
rS = peak response of orlistat from the Standard solution
CS = concentration of USP Orlistat RS in the Standard solution (mg/mL(IRA 1-Jan-2022))
CU = concentration of Orlistat in the Sample solution (mg/mL (IRA 1-Jan-2022))
F = relative response factor (see Table 3)
Acceptance criteria: See Table 3.
Table 3
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Orlistat related compound D | 0.94 | 1.0 | 0.2 |
| Orlistat | 1.00 | – | – |
| Orlistat open ring amideᵃ | 1.25 | 4.3 | 0.1 |
a (7S,8S,10S)-8-Hydroxy-7-{[(R)-1-phenylethyl]carbamoyl}henicosan-10-yl formyl-l-leucinate. (IRA 1-Jan-2022)
Change to read:
4.5 Limit of Orlistat Related Compound E
Buffer: 0.4 N borate solution, adjusted to a pH of 10.2
Derivatizing agent: o-Phthalaldehyde (OPA) solution. [Note—If unable to obtain commercially, the Derivatizing agent can be prepared as 1% each of 3-mercaptopropionic acid and o-phthalaldehyde in 0.4 M borate buffer solution.]
Solution A: Transfer 4.1 g of sodium acetate trihydrate and 40 mg of ethylenediaminetetraacetic acid (EDTA) into a 1-L volumetric flask.
Dissolve in 950 mL of water, and adjust with 0.1 N sodium hydroxide to a pH of 7.2. Dilute with water to volume, add 2.5 mL of tetrahydrofuran, and mix. Filter, and degas.
Solution B: Transfer 2.7 g of sodium acetate trihydrate and 40 mg of EDTA into a 1-L volumetric flask. Dissolve in 200 mL of water, and adjust with 0.1 N sodium hydroxide to a pH of 7.2. Add 800 mL of acetonitrile, filter, and degas.
Mobile phase: See Table 4.
Table 4
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 96.7 | 3.3 |
| 20 | 60 | 40 |
| 24 | 0 | 100 |
| 34 (IRA 1-Jan-2022) | 0 | 100 |
| 38 | 96.7 | 3.3 |
| 45 | 96.7 | 3.3 |
Standard solution: Transfer a weighed quantity of about 0.2 mg of USP Orlistat Related Compound E RS into a 20-mL headspace vial. Add 10mL of 4 N sodium hydroxide, and close the vial. Heat the vial to 100° for 1 h, then allow to cool to room temperature. Transfer 2 mL of the resulting solution into a 50-mL volumetric flask, and dilute with water to volume. To 0.5 mL of this solution add 2.0 mL of Buffer and 0.5 mL of Derivatizing agent.
Sample solution: Proceed as directed for the Standard solution, except use 25 mg of Orlistat to replace the 0.2 mg of USP Orlistat Related Compound E RS.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: Fluorescence 340 nm (excitation); 450 nm (emission)
Columns
Guard: 2.1-mm × 2-cm; 5-μm packing L1
Analytical: 2.1-mm × 20-cm; 5-μm packing L1
Flow rate: 0.5 mL/min
Injection volume: 20 μL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 6.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of orlistat related compound E in the portion of Orlistat taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of orlistat related compound E from the Sample solution
rS = peak response of orlistat related compound E from the Standard solution
CS = concentration of USP Orlistat Related Compound E RS in the Standard solution (mg/mL)
CU = concentration of Orlistat in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.2%
5 SPECIFIC TESTS
Optical Rotation 〈781S〉, Procedures, Specific Rotation
Sample solution: 30 mg/mL in dehydrated alcohol
Acceptance criteria: Between −48.0° and −51.0°, at 20°
Water Determination 〈921〉, Method I, Method Ic: NMT 0.2%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers between 2° and 8°.
Change to read:
USP Reference Standards 〈11〉
USP Orlistat RS
USP Orlistat Related Compound A RS
(3S,4S)-3-Hexyl-4-[(R)-2-hydroxytridecyl]oxetan-2-one. (IRA 1-Jan-2022)
C22H42O3 354.58 (IRA 1-Jan-2022)
USP Orlistat Related Compound B RS
Diisopropyl hydrazine-1,2-dicarboxylate.
C8H16N2O4 204.23 (IRA 1-Jan-2022)
USP Orlistat Related Compound C RS
Triphenylphosphine oxide.
C18H15OP 278.29
USP Orlistat Related Compound D Solution RS
Orlistat related compound D;
(3S,4R,6S)-3-Hexyl-2-oxo-6-undecyltetrahydro-2H-pyran-4-yl formyl-l-leucinate.
C29H53NO5 495.75 (IRA 1-Jan-2022)
USP Orlistat Related Compound E RS
(S)-1-[(2S,3S)-3-Hexyl-4-oxooxetan-2-yl]tridecan-2-yl formyl-l-isoleucinate. (IRA 1-Jan-2022)
C29H53NO5 495.75 (IRA 1-Jan-2022)


