Orbifloxacin
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
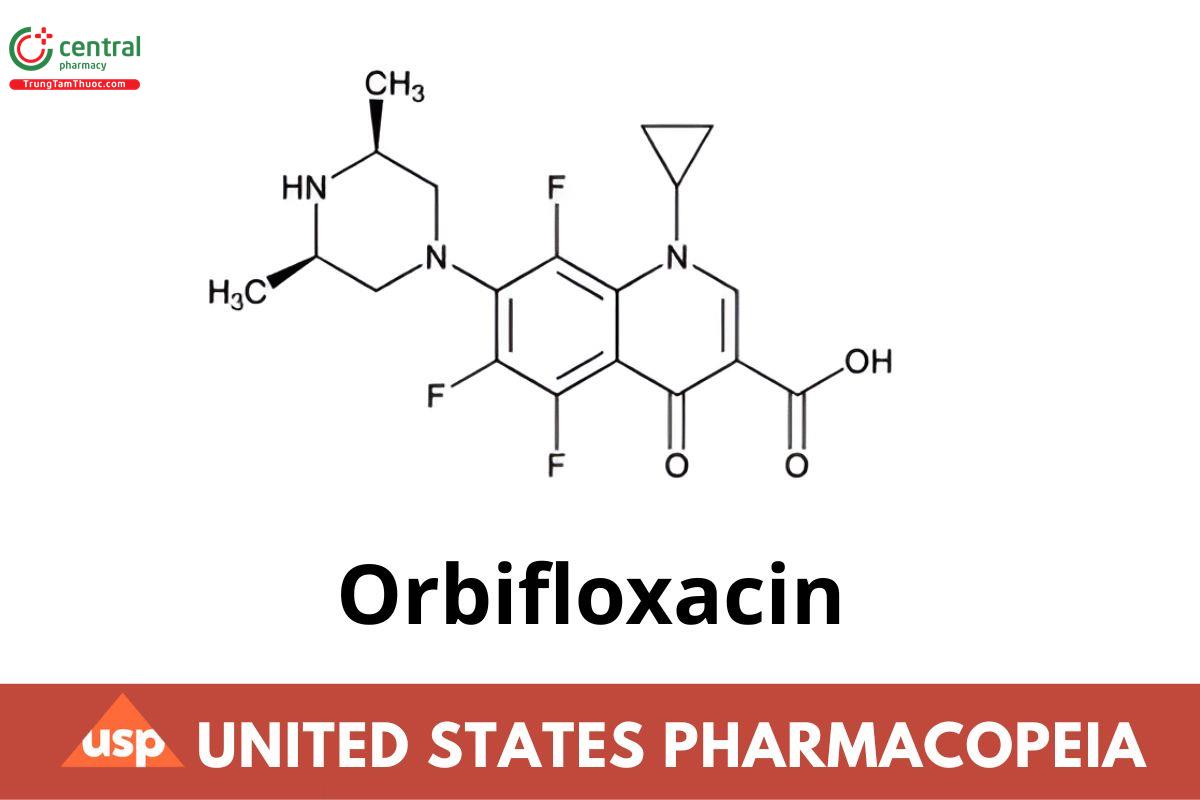
C19H20F3N3O3 395.38
1-Cyclopropyl-7-(cis-3,5-dimethyl-1-piperazinyl)-5,6,8-trifluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid CAS RN®: 113617-63-3; UNII: 660932TPY6.
Orbifloxacin contains not less than 98.5 percent and not more than 101.5 percent of C19H20F3N3O3, calculated on the anhydrous basis.
1 Packaging and storage
Preserve in well-closed containers. Store at room temperature.
USP Reference standards 〈11〉
USP Orbifloxacin RS
2 Identification
A: Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B: The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the
Standard preparation, as obtained in the Assay.
Change to read:
C: X-Ray Powder Diffraction 〈941〉 (CN 1-May-2022) The X-ray diffraction pattern conforms to that of USP Orbifloxacin RS, similarly determined.
Microbial enumeration tests 〈61〉- The total combined molds and yeasts count does not exceed 100 cfu per g.
pH 〈791〉: between 6.5 and 7.8, in a solution containing 10 mg per mL.
Water Determination, Method Ic 〈921〉: between 1.5% and 2.9%.
Residue on ignition 〈281〉: not more than 0.1%.
3 Related compounds
Buffer, Mobile phase, System suitability preparation, Standard preparation, and Chromatographic system - Prepare as directed in the Assay.
Standard solution - Dilute, quantitatively with Buffer, the Standard preparation to obtain a solution having a known concentration of about 0.00004 mg per mL.
Test solution - Transfer about 40 mg of Orbifloxacin, accurately weighed, to a 200-mL volumetric flask, dissolve in and dilute with Buffer to volume, and mix.
Chromatographic system (see Chromatography 〈621〉) - Inject the Buffer as directed for Procedure to verify that there are no interfering peaks.
Procedure - Separately inject equal volumes (about 10 μL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the area responses for the major peaks. Calculate the percentage of related compounds in the portion of Orbifloxacin taken by the formula:
20,000(CS)(ri/rS)(1/F)(1/W)
in which CS is the concentration, in mg per mL, of orbifloxacin in the Standard solution; ri is the peak area response for each impurity obtained from the Test solution; rS is the peak area response for the orbifloxacin peak obtained from the Standard solution; F is the relative response factor for each impurity, as presented in Table 1; and W is the sample weight taken to prepare the Test solution (mg).
Table 1
| Component / Impurity | Approximate Relative Retention Time | Relative Response Factor (F) | Limit % |
|---|---|---|---|
| cis, cis-1-Cyclopropyl-5,7-bis(3,5-dimethyl-1-piperazinyl)-6,8-difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid | 0.5 | 0.36 | NMT 0.2 |
| cis-1-Cyclopropyl-7-(3,5-dimethyl-1-piperazinyl)-5,6,8-trifluoro-4(1H)-quinolinone | 0.65 | 0.27 | NMT 0.2 |
| 7-[(2-Aminopropyl)amino]-1-cyclopropyl-5,6-difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid | 0.75 | 0.49 | NMT 0.2 |
| Orbifloxacin | 1.0 | 1.00 | – |
| 1-Cyclopropyl-7-(3,5-dimethyl-1-piperazinyl)-6,8-difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid | 1.4 | 0.84 | NMT 0.2 |
| cis-1-Cyclopropyl-7-(3,5-dimethyl-1-piperazinyl)-6,8-difluoro-1,4-dihydro-5-hydroxy-4-oxo-3-quinolinecarboxylic acid | 2.7 | 0.73 | NMT 0.2 |
| cis-1-Cyclopropyl-5-(3,5-dimethyl-1-piperazinyl)-6,7,8-trifluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid | 3.6 | 0.11 | NMT 0.2 |
| 1-Cyclopropyl-5,6,7,8-tetrafluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid | 6.8 | 0.16 | NMT 0.2 |
| Unknown | – | 1.0 | – |
| Total known and unknown | – | – | NMT 0.4 |
4 Assay
Buffer-n a 2-L flask, dissolve about 11.8 g of sodium citrate in 1600 mL of water, and mix. Add 180 mL of acetic acid, and mix. Adjust with 6 N sodium hydroxide to a pH of 3.5, dilute with water to about 2 L, and mix.
Mobile phase - Prepare a filtered and degassed mixture of Buffer, methanol, and dioxane (86:11:4). Make adjustments if necessary (see System Suitability under Chromatography 〈621〉).
Standard stock preparation - Dissolve in Buffer an accurately weighed quantity of USP Orbifloxacin RS to obtain a solution having a known concentration of about 0.2 mg per mL.
Standard preparation - Accurately transfer a quantity of Standard stock preparation, and dilute with Buffer to obtain a solution having a known concentration of about 0.02 mg per mL.
System suitability preparation - Dissolve about 40 mg of methyl 4-aminobenzoate in 2 mL of methanol, and dilute with Buffer to 200 mL. Pipet 10.0 mL of this solution and 10.0 mL of Standard stock preparation into a 100-mL volumetric flask. Dilute with Buffer to volume, and mix.
Assay preparation - Transfer about 40 mg of Orbifloxacin accurately weighed, to a 200-mL volumetric flask, dissolve in and dilute with Buffer to volume, and mix. Dilute with Buffer an aliquot of the resulting solution to obtain a solution having a known concentration of about 0.02 mg per mL.
Chromatographic system (see Chromatography 〈621〉) - The liquid chromatograph is equipped with a 290-nm detector and 4.6-mm × 3.0-cm column that contains 3-μm packing L1. The flow rate is about 1.0 mL per minute. Prior to injecting the System suitability preparation, flush the column with approximately 50 mL of a mixture of acetonitrile and water (9:1). Chromatograph the System suitability preparation, and record the peak response as directed for Procedure: the relative retention times are about 1.3 for methyl 4-aminobenzoate and 1.0 for orbifloxacin; the resolution, R, between methyl 4-aminobenzoate and orbifloxacin is not less than 2; the tailing factor is not more than 1.8; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure - Separately inject equal volumes (about 10 μL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatographs, and measure the area responses for the major peaks. Calculate the quantity, in mg, of C19H20F3N3O3 in the portion of Orbifloxacin taken by the formula:
2000C(rU/rS)
in which C is the concentration, in mg per mL, of USP Orbifloxacin RS in the Standard preparation; and rU and rS are the peak area responses obtained from the Assay preparation and the Standard preparation, respectively.


