Ondansetron
If you find any inaccurate information, please let us know by providing your feedback here
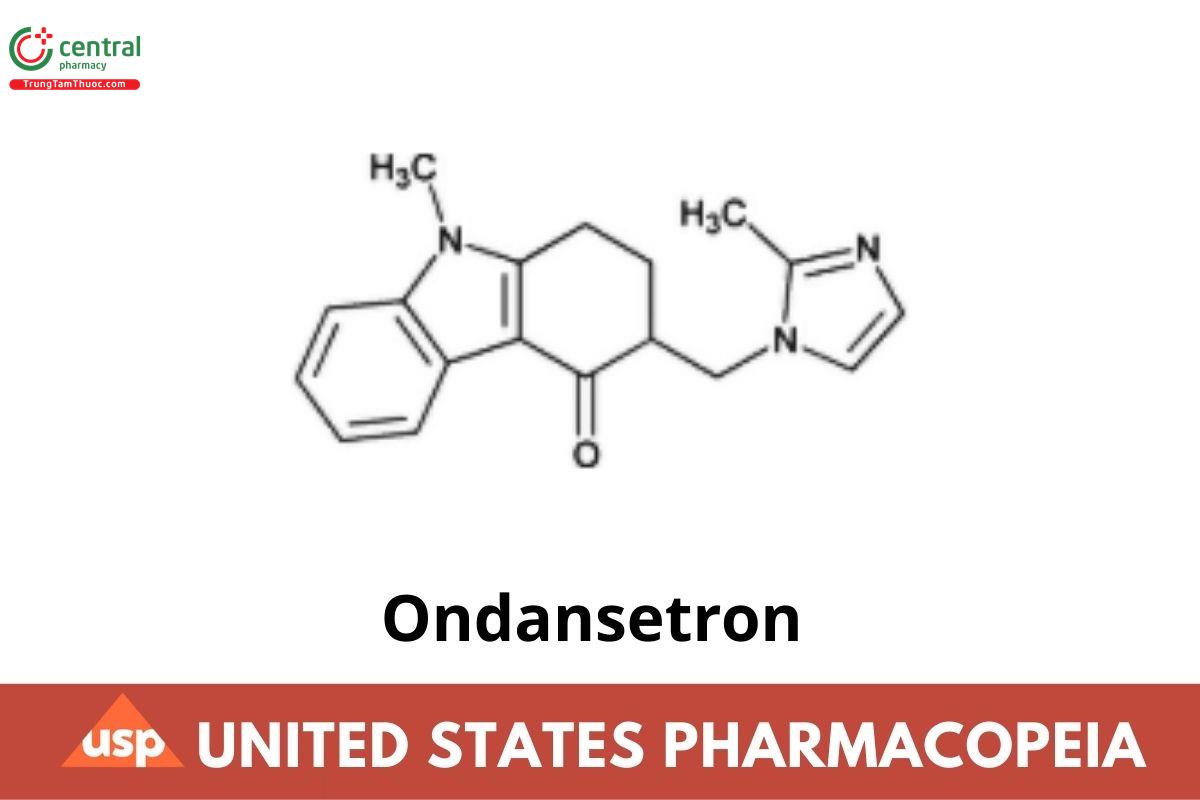
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Ondansetron contains NLT 98.0% and NMT 102.0% of ondansetron (C₁₈H₁₉N₃O), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: ▲197A or▲ (USP 1-Aug-2024) 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Change to read:
Procedure
▲Solution A:** 2.4 g/L of monobasic sodium phosphate, anhydrous and 0.6 g/L of sodium 1-heptanesulfonate in water. Adjust with 0.5 N sodium hydroxide to a pH of 5.4.
Solution B: Acetonitrile
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 95 | 5 |
| 5 | 95 | 5 |
| 8 | 80 | 20 |
| 25 | 35 | 65 |
| 27 | 30 | 70 |
| 33 | 30 | 70 |
| 35 | 95 | 5 |
| 40 | 95 | 5 |
Diluent: Acetonitrile and water (30:70). To each liter of this solution add 1 mL of formic acid.
Standard solution: 0.1 mg/mL of USP Ondansetron RS in Diluent
Sample solution: 0.1 mg/mL of Ondansetron in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 216 nm
Column: 4.6-mm × 25-cm; 5-μm packing L11
Temperatures
Autosampler: 15°
Column: 40°
Flow rate: 1 mL/min
Injection volume: 10 μL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 0.73%▲ (USP 1-Aug-2024)
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of ondansetron (C₁₈H₁₉N₃O) in the portion of Ondansetron taken:
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × 100
rᵤ = peak response of ondansetron from the Sample solution
rₛ = peak response of ondansetron from the Standard solution
Cₛ = concentration of USP Ondansetron RS in the Standard solution (mg/mL)
Cᵤ = concentration of Ondansetron in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Change to read:
Chloride and Sulfate 〈221〉, Chloride
▲Sample: 1 g
Acceptance criteria: The turbidity produced in the Sample does not exceed that produced in 0.3 mL of 0.020 N hydrochloric acid (NMT 0.02%).▲ (USP 1-Aug-2024)
Delete the following:
▲• Limit of Ondansetron Related Compound D▲ (USP 1-Aug-2024)
Change to read:
Organic Impurities
▲Solution A, Solution B, Mobile phase, Diluent, and Chromatographic system:** Proceed as directed in the Assay.
System suitability solution: 1 mg/mL of USP Ondansetron RS and 0.001 mg/mL of USP Ondansetron Related Compound G in Diluent
Standard solution: 0.001 mg/mL each of USP Ondansetron RS and USP Ondansetron Related Compound D RS in Diluent
Sensitivity solution: 0.5 μg/mL each of USP Ondansetron RS and USP Ondansetron Related Compound D RS from the Standard solution in Diluent
Sample solution: 1.0 mg/mL of Ondansetron in Diluent
System suitability
Samples: System suitability solution, Standard solution, and Sensitivity solution
[Note—The relative retention times in Table 2 are provided as information that could aid in peak assignment.]
| Name | Relative Retention Time |
|---|---|
| Imidazoleᵃ | 0.28 |
| Ondansetron related compound Fᵇ | 0.33 |
| Ondansetron related compound Aᶜ | 0.94 |
| Ondansetron | 1.00 |
| Ondansetron related compound G | 1.04 |
| Ondansetron related compound Cᵈ | 1.11 |
| Methylene bisondansetronᵉ | 1.13 |
| Ondansetron related compound D | 1.2 |
ᵃ 1H-Imidazole.
ᵇ 2-Methyl-1H-imidazole.
ᶜ 3-[(Dimethylamino)methyl]-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one.
ᵈ 9-Methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one.
ᵉ Disregard the peak corresponding to methylene bisondansetron. Quantified in the test for Limit of Methylene Bisondansetron.
Suitability requirements
Resolution: NLT 2.0 between ondansetron and ondansetron related compound G
Relative standard deviation: NMT 5.0% for ondansetron, Standard solution
Signal-to-noise ratio: NLT 10 for ondansetron, Sensitivity solution
Analysis
Calculate the percentage of ondansetron related compound D in the portion of Ondansetron taken:
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × 100
Calculate the percentage of any unspecified impurity in the portion of Ondansetron taken:
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × 100
Acceptance criteria: See Table 3.
The reporting threshold is 0.05%.
Table 3
| Name | Acceptance Criteria, NMT (%) |
|---|---|
| Ondansetron related compound D | 0.10 |
| Any unspecified impurity | 0.1 |
| Total impurities | 0.5 |
▲ (USP 1-Aug-2024)
Add the following:
▲• Limit of Methylene Bisondansetron (If Present)
Buffer: 2.4 g/L of monobasic sodium phosphate, anhydrous in water. Adjust with 0.5 N sodium hydroxide to a pH of 6.5.
Mobile phase: Acetonitrile and Buffer (65:35)
Peak identification solution: 1 mg/mL of USP Ondansetron Resolution Mixture RS in Mobile phase
Standard solution: 0.002 mg/mL of USP Ondansetron RS in Mobile phase
Sensitivity solution: 0.001 mg/mL of USP Ondansetron RS from the Standard solution in Mobile phase
Sample solution: 1.0 mg/mL of Ondansetron in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 216 nm
Column: 4.6-mm × 25-cm; 5-μm packing L10
Temperatures
Autosampler: 15°
Column: 35°
Flow rate: 1.5 mL/min
Injection volume: 20 μL
Run time: NLT 5 times the retention time of ondansetron
System suitability
Samples: Standard solution and Sensitivity solution
[Note—The relative retention times for ondansetron and methylene bisondansetron are 1.0 and 3.7, respectively.]
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 5.0%
Signal-to-noise ratio: NLT 10
Analysis
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × (1/F) × 100
F = relative response factor, 0.63
Acceptance criteria: NMT 0.4%▲ (USP 1-Aug-2024)
5 SPECIFIC TESTS
Water Determination 〈921〉, Method I, Method Ia: NMT 3.0%
Add the following:
▲Bacterial Endotoxins Test 〈85〉: Where the label states that Ondansetron must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement under the relevant dosage form monograph(s) in which Ondansetron is used can be met.▲ (USP 1-Aug-2024)
Add the following:
▲Microbial Enumeration Tests 〈61〉: The total aerobic microbial count does not exceed 10² cfu/g and the total combined molds and yeast count does not exceed 50 cfu/g.▲ (USP 1-Aug-2024)
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers at room temperature.
Add the following:
▲Labeling: Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.▲ (USP 1-Aug-2024)
Change to read:
USP Reference Standards 〈11〉
USP Ondansetron RS ▲▲ (USP 1-Aug-2024)
USP Ondansetron Related Compound D RS
9-Methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one.
▲C₁₄H₁₃NO 211.26
USP Ondansetron Related Compound G
3-[(1H-Imidazol-1-yl)methyl]-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one.
C₁₇H₁₇N₃O 279.34
USP Ondansetron Resolution Mixture RS
Contains a mixture of the following 3 compounds:
Ondansetron hydrochloride.
Ondansetron related compound A;
3-[(Dimethylamino)methyl]-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one hydrochloride.
C₁₈H₂₂N₂O · HCl 292.81
Methylene bisondansetron;
6,6'-Methylenebis{9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4H-carbazol-4-one}.
C₃₆H₃₈N₆O₂ 598.75▲ (USP 1-Aug-2024)


