Olmesartan Medoxomil
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
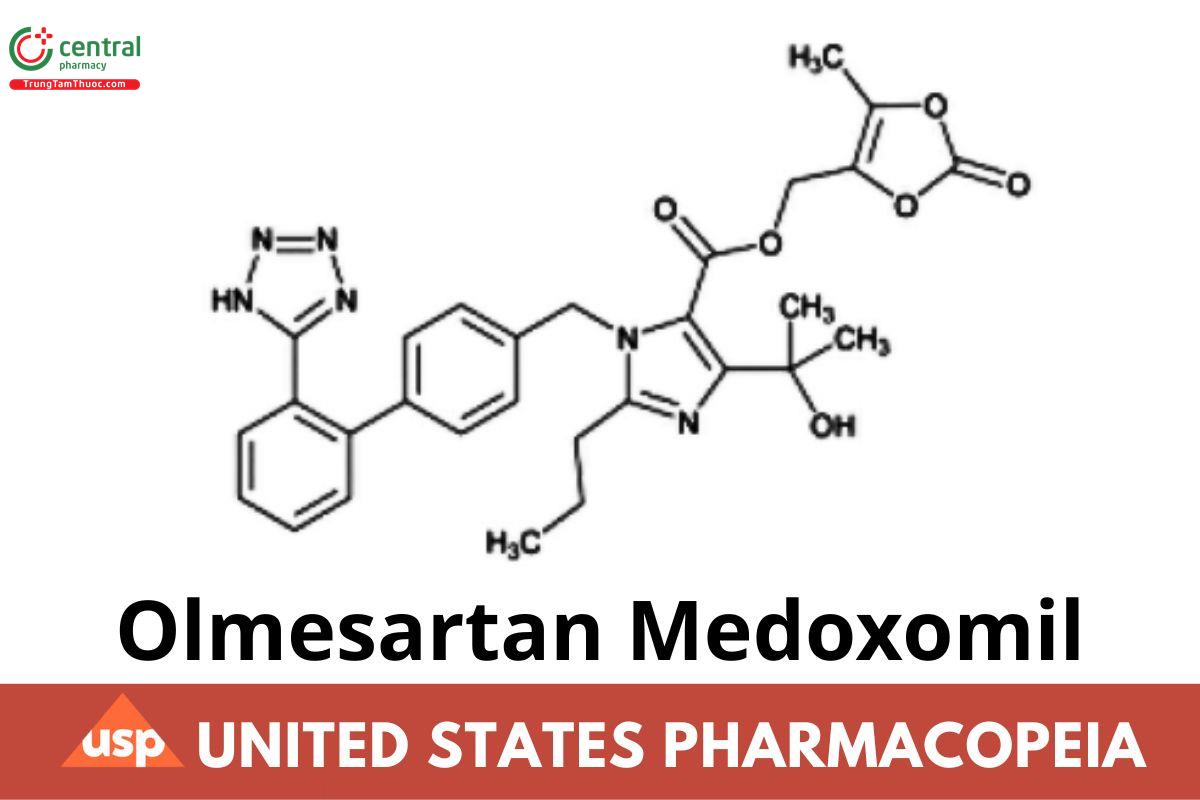
C29H30N6O6 558.59
1H-Imidazole-5-carboxylic acid, 4-(1-hydroxy-1-methyl ethyl)-2-propyl-1-[[2′-(1H-tetrazol-5-yl) [1,1′-biphenyl]-4-yl]methyl]-, (5-methyl-2-oxo-1,3- dioxol-4-yl)methyl ester; (5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 1-{[2′-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-4-(2-hydroxypropan-2-yl)-2-propyl-1H imidazole-5-carboxylate CAS RN®: 144689-63-4; UNII: 6M97XTV3HD.
1 DEFINITION
Olmesartan Medoxomil contains NLT 98.5% and NMT 101.5% of C29H30N6O6, calculated on the anhydrous and solvent-free basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B. The ratio of the retention time of the major peak to that of the internal standard of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
[Note—The Standard solution and Sample solution are stable for 24 h at 5°.]
Diluted phosphoric acid: 0.2% phosphoric acid
Buffer: 0.015 M monobasic potassium phosphate. Adjust the solution with Diluted phosphoric acid (w/v) to a pH of 3.4. Mobile phase: Acetonitrile and Buffer (17:33)
Diluent 1: Acetonitrile and water (4:1)
Diluent 2: Acetonitrile and water (2:3)
Internal standard solution: 0.5 mg/mL of 4-hydroxybenzoic acid isobutyl ester in Diluent 2. [Note—This solution is stable for 1 month at room temperature.]
Standard stock solution: 1 mg/mL of USP Olmesartan Medoxomil RS in Diluent 1
Standard solution: 0.05 mg/mL of USP Olmesartan Medoxomil RS from the Standard stock solution and 0.025 mg/mL of p-hydroxybenzoic acid isobutyl ester from the Internal standard solution in Diluent 2
Sample stock solution: 1 mg/mL of Olmesartan Medoxomil in Diluent 1
Sample solution: 0.05 mg/mL of Olmesartan Medoxomil from the Sample stock solution and 0.025 mg/mL of p-hydroxybenzoic acid isobutyl ester from the Internal standard solution in Diluent 2
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 250 nm
Column: 4.6-mm × 15-cm; 5-µm packing L1
Column temperature: 40°
Flow rate: 1 mL/min
Injection size: 10 µL
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 4 between olmesartan medoxomil and p-hydroxybenzoic acid isobutyl ester
Relative standard deviation: NMT 0.5% for the peak ratio of olmesartan medoxomil and the internal standard Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of olmesartan medoxomil in the portion taken:
Result = (RU/RS) × (CS/CU) × 100
RU = ratio of the peak areas of olmesartan medoxomil and p-hydroxybenzoic acid isobutyl ester from the Sample solution
RS = ratio of the peak areas of olmesartan medoxomil and p-hydroxybenzoic acid isobutyl ester from the Standard solution
CS = concentration of USP Olmesartan Medoxomil RS in the Standard solution (mg/mL)
CU = concentration of Olmesartan Medoxomil in the Sample solution (mg/mL)
Acceptance criteria: 98.5%–101.5% on the anhydrous and solvent-free basis
4 IMPURITIES
Inorganic Impurities
Residue on Ignition 〈281〉: NMT 0.1%. [Note—The ignition temperature range is 450° to 550°.]
Organic Impurities
Procedure
Buffer: Prepare as directed in the Assay.
Solution A: Acetonitrile and Buffer (1:4)
Solution B: Acetonitrile and Buffer (4:1)
Mobile phase: See the gradient table below.
Time (min) | Solution A (%) | Solution B (%) |
0 | 75 | 25 |
10 | 75 | 25 |
35 | 0 | 100 |
45 | 0 | 100 |
System suitability solution: 0.01 mg/mL each of USP Olmesartan Medoxomil RS and USP Olmesartan Medoxomil Related Compound A RS in acetonitrile
Standard solution: 0.01 mg/mL of USP Olmesartan Medoxomil RS in acetonitrile
Sample solution: 1 mg/mL of Olmesartan Medoxomil in acetonitrile
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
[Note—A guard column of 4.6-mm × 5-cm of packing L7 may be used.]
Mode: LC
Detector: UV 250 nm
Column: 4.6-mm × 10-cm; 3.5-µm packing L7
Column temperature: 40°
Flow rate: 1 mL/min
Injection size: 10 µL
System suitability
Suitability requirements
Sample: System suitability solution
Resolution: NLT 5 between olmesartan medoxomil and olmesartan medoxomil related compound A
Relative standard deviation: NMT 2.0% for the olmesartan medoxomil peak
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Olmesartan Medoxomil taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of olmesartan medoxomil from the Standard solution
CS = concentration of USP Olmesartan Medoxomil RS in the Standard solution (mg/mL)
CU = concentration of Olmesartan Medoxomil in the Sample solution (mg/mL)
F = relative response factor (see the Impurity Table)
Acceptance criteria
Individual impurities: See the Impurity Table.
Total impurities: NMT 1.3%. [Note—Disregard any peak below 0.05%.]
Impurity Table
Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
Olmesartana | 0.2 | 1.0 | 0.5 |
Olmesartan medoxomil related compound Ab | 0.7 | 1.6 | 0.1 |
Olmesartan medoxomil | 1.0 | 1.0 | — |
Olmesartan dimerc | 1.6 | 1.0 | 0.6 |
Olenic impurityd | 3.4 | 0.7 | 0.1 |
Any other individual unspecified impurity | — | 1.0 | 0.1 |
a 1-{[2′-(1H-Tetrazol-5-yl)biphenyl-4-yl]methyl}-4-(2-hydroxypropan-2-yl)-2-propyl-1H-imidazole-5-carboxylic acid.
b 1-{[2′-(1H-Tetrazol-5-yl)biphenyl-4-yl]methyl}-4,4-dimethyl-2-propyl-1H-furo[3,4-d]imidazol-6(4H)-one.
c(5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 1-((2′-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-4-(prop-1-en-2-yl)-2-propyl-1H-imidazole-5- carboxylate.
d(5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 4-(2-hydroxypropan-2-yl)-2-propyl-1-((2′-(2-trityl-2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-1H imidazole-5-carboxylate.
5 SPECIFIC TESTS
Limit of Acetone (if present)
Internal standard solution: 1% solution of 1-butanol in Dimethyl sulfoxide. [Note—This solution is stable for 1 month at room temperature.] Standard solution: 0.37 µL/mL of acetone and 2 µL/mL of 1-butanol from the Internal standard solution in dimethylsulfoxide. [Note—This solution is stable for 8 h at room temperature.]
Sample solution: 25 mg/mL of Olmesartan Medoxomil and 2 µL/mL of 1-butanol from the Internal standard solution in dimethylsulfoxide. [Note—This solution is stable for 8 h at room temperature.]
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 30-m × 0.53-mm column bonded with a 1-µm lm of phase G14
Column temperature: See the temperature program table below.
Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
50 | 0 | 50 | 5 |
50 | 10 | 180 | 5 |
Injection port temperature: 200°
Detector temperature: 200°
Autosampler temperature: 80°
Carrier gas: Helium
Flow rate: 4 mL/min. [Note—Adjust the ow rate so that the retention time of acetone is 2.5 min.]
Injection size: 1 mL
Split ratio: 5:1
System suitability
Sample: Standard solution. [Note—Allow the samples to stand for 30 min in the autosampler at 80°.]
Suitability requirements
Resolution: NLT 60 between the acetone and 1-butanol peaks
Relative standard deviation: NMT 5.0% for the peak area ratio of acetone and 1-butanol
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of acetone in the portion of Olmesartan Medoxomil taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of acetone from the Sample solution
rS = peak response of acetone from the Standard solution
CS = concentration of acetone in the Standard solution (mg/mL)
CU = concentration of Olmesartan Medoxomil in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.6%
Water Determination, Method Ic〈921〉: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers, protect from moisture, and store below 25°.
USP Reference Standards 〈11〉
USP Olmesartan Medoxomil RS
USP Olmesartan Medoxomil Related Compound A RS
1-{[2′-(1H-Tetrazol-5-yl)biphenyl-4-yl]methyl}-4,4-dimethyl-2-propyl-1H-furo[3,4-d]imidazol-6(4H)-one.
C24H24N6O2 428.49


