Octreotide Acetate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
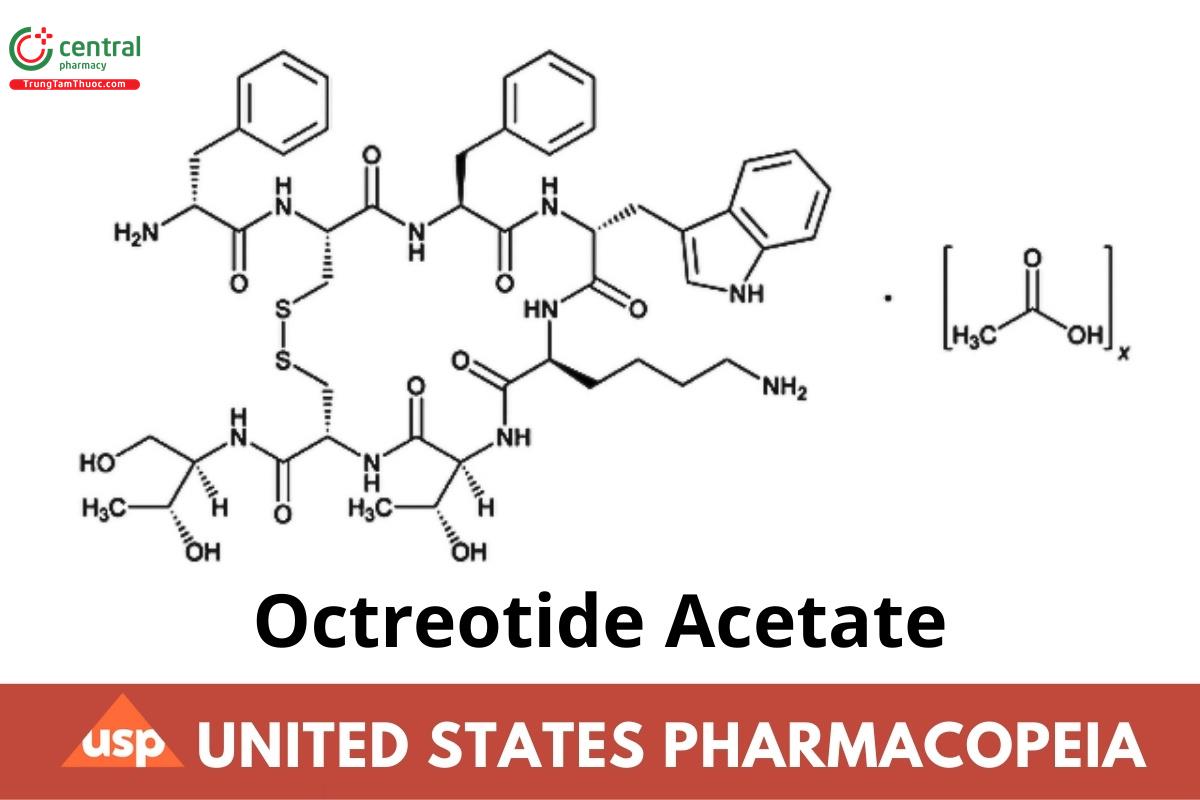
C49H66N10O10S2 · xC2H4O2 1019 (as free base)
l-Cysteinamide, d-phenylalanyl-l-cysteinyl-l-phenylalanyl-d-tryptophyl-l-lysyl-l-threonyl-N-[2-hydroxy-1-(hydroxymethyl)propyl]-, cyclic (2→7)-disulfide, [R-(R*,R*)]-, acetate (salt);
d-Phenylalanyl-l-cysteinyl-l-phenylalanyl-d-tryptophyl-l-lysyl-l-threonyl-N-[(1R,2R)-2-hydroxy-1-(hydroxymethyl)propyl]-l-cysteinamide cyclic (2→7)-disulfide acetate (salt);
d-Phenylalanyl-l-hemicystyl-l-phenylalanyl-d-tryptophyl-l-lysyl-l-threonyl-l-hemicystyl-l-threoninol cyclic (2→7)-disulfide acetate (salt)
CAS RN®: 79517-01-4; UNII: 75R0U2568I
1 DEFINITION
Octreotide Acetate is a long-acting synthetic octapeptide with pharmacologic properties mimicking those of the natural hormone Somatostatin.
It contains NLT 95.0% and NMT 105.0% of octreotide (C49H66N10O10S2), calculated on the anhydrous, acetic acid-free basis.
2 IDENTIFICATION
2.1 A. HPLC
Solution A, Solution B, Mobile phase, System suitability solution, Standard solution, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Identification sample solution: Mix an equal volume of the Standard solution and the Sample solution.
Analysis
Samples: Identification sample solution, Standard solution, and Sample solution
Examine the chromatograms of the Identification sample solution, Standard solution, and Sample solution.
Acceptance criteria: The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, and the major peak of the Identification sample solution elutes as a single peak.
2.2 B. Amino Acid Analysis
[Note—The following method is given for informational purposes; any validated Amino acid analysis method can be used.]
Diluent: 0.1 M hydrochloric acid
Standard amino acid mixture: 2500 nmol/mL each of Lys, His, NH₃, Arg, Asp, Thr, Ser, Glu, Pro, Gly, Ala, Val, Met, Ile, Leu, Tyr, Phe, and 1250 nmol/mL of Cys-Cys in Diluent
Standard tryptophan solution: 0.2042 mg/mL of tryptophan in Diluent. This solution contains 1000 nmol/mL of Trp.
Standard threoninol solution: 0.1052 mg/mL of Thr-ol in Diluent. This solution contains 1000 nmol/mL Thr-ol.
[Note—1000 nmol/mL = 1 mM]
Standard solution: Diluent, Standard amino acid mixture, Standard tryptophan solution, and Standard threoninol solution (76:4:10:10). The concentration is 100.0 nmol/mL for each amino acid.
Sample solution A: Weigh 1.00–1.50 mg of Octreotide Acetate into a 5-mL ampule. Add 1000 µL of Diluent and 1.2 mL of 30% (w/w) hydrochloric acid, cool the ampule in dry ice, evacuate, and seal by melting. Heat the ampule for 16 h at 115°. Allow to cool and transfer the contents of the ampule quantitatively into a 25-mL round-bottomed flask, rinse with Diluent, and evaporate to dryness on a rotary evaporator. Dissolve the residue in 10.00 mL of Diluent.
Sample solution B: Weigh 1.00–1.50 mg of Octreotide Acetate into a 5-mL ampule. Add 1000 µL of Diluent and 1.2 mL of 30% (w/w) hydrochloric acid with 1% (v/v) thioglycolic acid, cool the ampule in dry ice, evacuate, and seal by melting. Heat the ampule for 16 h in a heating block at 115°. Allow to cool and transfer the contents of the ampule quantitatively into a 25-mL round-bottomed flask, rinse with Diluent, and evaporate to dryness on a rotary evaporator. Dissolve the residue in 10.00 mL of Diluent.
Analysis
Samples: Standard solution, Sample solution A, and Sample solution B
Standardize the instrument with the Standard solution. Inject a suitable volume of Standard solution, Sample solution A, and Sample solution B. Evaluate the peak areas of each amino acid found in relation to the peak areas of the respective amino acids in the Standard solution, express the content of each amino acid in nmoles.
Calculate A, the average number of nmoles of the amino acids found to be stable under hydrolysis conditions (three stable amino acids—2 Phe and 1 Lys) taken:
A = NT/3
NT = total nmoles of the stable amino acids
Calculate the ratio of the amino acids taken:
Result = NE/A
NE = nmoles of each amino acid
[Note—For Trp use only data obtained with Sample solution B. For Cys use only data obtained with Sample solution A.]
Acceptance criteria: See Table 1.
Table 1
| Name | Acceptance Criteria |
| Thr | 0.7–1.1 |
| Lys | 0.9–1.3 |
| Phe | 1.8–2.2 |
| Trp | 0.4–1.1 |
| Cys | 1.0–2.2 |
| Thr-ol | 0.6–1.3 |
3 ASSAY
3.1 Procedure
Solution A: 0.02% (v/v) of trifluoroacetic acid in water
Solution B: Acetonitrile
Mobile phase: See Table 2.
Table 2
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 90 | 10 |
| 25 | 65 | 35 |
| 30 | 10 | 90 |
| 35 | 10 | 90 |
| 40 | 90 | 10 |
| 45 | 90 | 10 |
System suitability solution: 0.5 mg/mL of USP Octreotide Acetate RS and 0.2 mg/mL of USP Octreotide Non-Cyclic System Suitability Marker RS in Solution A
Standard solution: 0.5 mg/mL of USP Octreotide Acetate RS in Solution A
Sample solution: 0.5 mg/mL of Octreotide Acetate in Solution A.
[Note—Place Octreotide Acetate in a desiccator containing saturated sodium chloride solution for at least 30 min before weighing. Determine the water content by suitable analysis.]
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 25-cm; 4-µm packing L87
Column temperature: 40°
Flow rate: 1 mL/min
Injection volume: 10 µL
System suitability
Samples: System suitability solution and Standard solution
[Note—The retention times for octreotide and non-cyclic octreotide in the System suitability solution are about 16.5 and 18.5 min, respectively.]
Suitability requirements
Resolution: NLT 2.0 between octreotide and non-cyclic octreotide, System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of octreotide (C49H66N10O10S2) in the portion of Octreotide Acetate taken:
Result = (rU/rS) × (CS/CU) × [100 / (100 − W − Ac)] × 100
rU = peak response of octreotide from the Sample solution
rS = peak response of octreotide from the Standard solution
CS = concentration of USP Octreotide Acetate RS in the Standard solution (mg/mL)
CU = concentration of Octreotide Acetate in the Sample solution (mg/mL)
W = water content in the Octreotide Acetate sample (%)
Ac = acetic acid content in the Octreotide Acetate sample (%)
Acceptance criteria: 95.0%–105.0% on the anhydrous, acetic acid-free basis
4 OTHER COMPONENTS
Acetic Acid in Peptides 〈503〉: 5.0%–12.8%
5 PRODUCT-RELATED SUBSTANCES AND IMPURITIES
5.1 Octreotide Acetate Related Compounds
[Note—Manufacturers should determine the suitability of their related substances method for their process-related and degradation impurities. For any impurity peak above the limit for unspecified impurity peaks, identification and appropriate qualification is required.]
Solution A, Solution B, Mobile phase, System suitability solution, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Analysis
Sample: Sample solution
Calculate the percentage of each impurity taken, disregarding any peak with a retention time of less than 5 min and any peak with an area less than 0.1% of the main peak:
Result = (rU/rT) × 100
rU = peak response of each impurity from the Sample solution
rT = sum of the peak responses from the Sample solution, excluding those of the solvent peaks
Acceptance criteria: See Table 3.
Table 3
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Acetyl-Lys⁵-octreotideᵃ | 1.4 | 0.5 |
| Acetyl-Phe¹-octreotideᵇ | 1.5 | 0.5 |
| Unspecified impurity | — | 0.5 |
| Total impurities | — | 2.0 |
ᵃ D-Phenylalanyl-L-hemicystyl-L-phenylalanyl-D-tryptophyl-(N-acetyl)-L-lysyl-L-threonyl-L-hemicystyl-L-threoninol cyclic (2→7)-disulfide.
ᵇ (N-Acetyl)-D-Phenylalanyl-L-hemicystyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-L-hemicystyl-L-threoninol cyclic (2→7)-disulfide.
6 PROCESS-RELATED IMPURITIES
Trifluoroacetic Acid (TFA) in Peptides 〈503.1〉: NMT 0.25%.
[Note—Perform this test if trifluoroacetic acid is used in the manufacturing process.]
7 SPECIFIC TESTS
Water Determination 〈921〉, Method I: NMT 10.0%
Bacterial Endotoxins Test 〈85〉: NMT 466 USP Endotoxin Units/mg of octreotide acetate
Microbial Enumeration Tests 〈61〉 and Tests for Specified Microorganisms 〈62〉: The total aerobic microbial count is NMT 100 cfu/g. The total yeast and mold count is NMT 100 cfu/g.
8 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in air-tight containers. Store at a temperature of 2°–8°, protected from light.
USP Reference Standards 〈11〉
USP Endotoxin RS
USP Octreotide Acetate RS
USP Octreotide Non-Cyclic System Suitability Marker RS


