Octisalate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
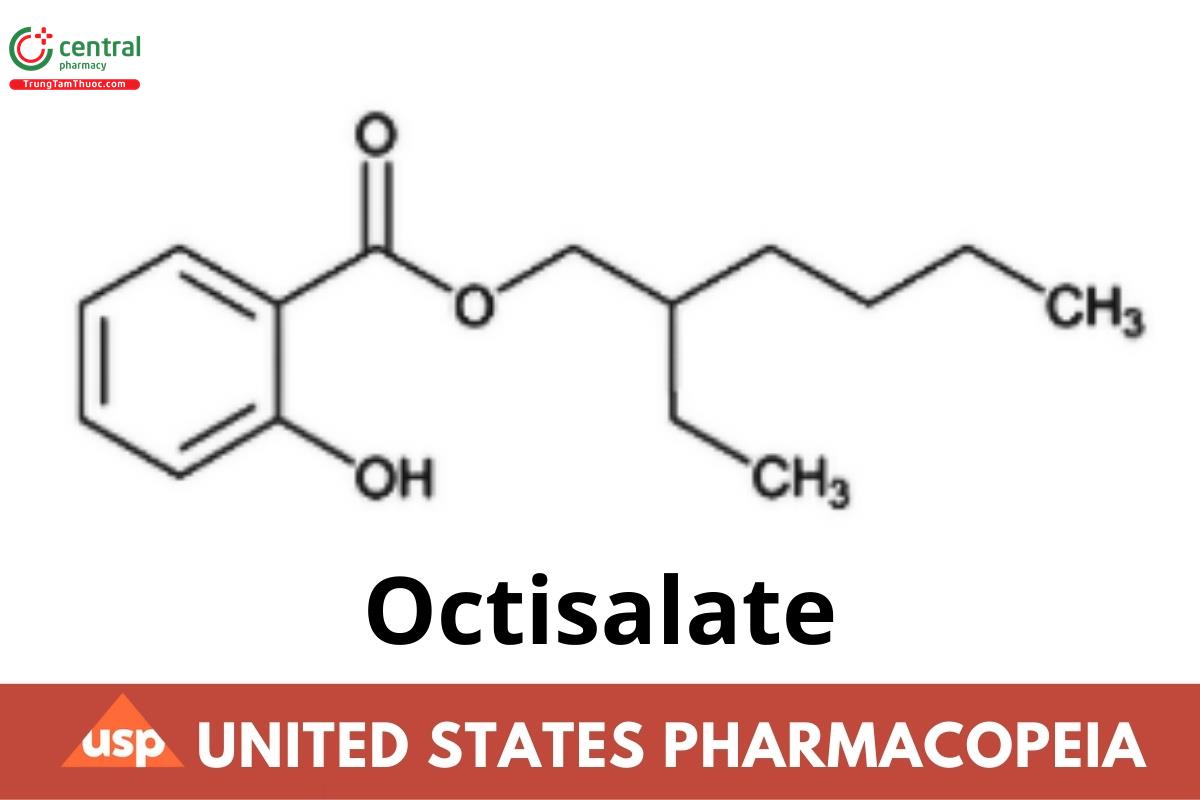
C15H22O3 250.33
Benzoic acid, 2-hydroxy-, 2-ethylhexyl ester;
2-Ethylhexyl salicylate
CAS RN®: 118-60-5; UNII: 4X49Y0596W
1 DEFINITION
Octisalate contains NLT 95.0% and NMT 105.0% of octisalate (C15H22O3), calculated on the as-is basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A or 197F (CN 1-May-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 Procedure
Standard solution: 20 mg/mL of USP Octisalate RS in tert-butyl methyl ether
Sample solution: 20 mg/mL of Octisalate in tert-butyl methyl ether
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm × 25-m; coated with a 0.1-µm film of phase G1
Temperatures
Injector: 240°
Detector: 260°
Column: See Table 1.
Table 1
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 60 | 8 | 240 | 10 |
Carrier gas: Helium
Flow rate: 6 mL/min
Injection volume: 1 µL
Injection type: Split; split ratio 50:1.
[Note—Split ratio can be modified to optimize the performance.]
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 1.0 between octisalate and any other peak
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of octisalate (C15H22O3) in the portion of Octisalate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Octisalate RS in the Standard solution (mg/mL)
CU = concentration of Octisalate in the Sample solution (mg/mL)
Acceptance criteria: 95.0%–105.0% on the as-is basis
4 IMPURITIES
4.1 Organic Impurities
Standard solution, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Octisalate taken:
Result = (rU/rT) × 100
rU = peak response of each impurity from the Sample solution
rT = sum of all the peak responses from the Sample solution
Acceptance criteria
Individual impurities: NMT 0.5%
Total impurities: NMT 2.0%
5 SPECIFIC TESTS
Specific Gravity 〈841〉: 1.011–1.016
Refractive Index 〈831〉: 1.500–1.503 at 20°
Acidity
Sample solution: 5.0 mL of measured Octisalate
Titrimetric system
Mode: Direct titration
Titrant: 0.1 N sodium hydroxide
Endpoint detection: Visual
Analysis: Transfer 50 mL of alcohol to a suitable container, add 1 mL of phenol red TS, and add sufficient Titrant to obtain a persistent pink color. Transfer 50 mL of this solution to a suitable container, add the Sample solution, mix, and titrate with Titrant.
Acceptance criteria: NMT 0.2 mL of 0.1 N sodium hydroxide per milliliter of Octisalate is required for neutralization.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
USP Reference Standards 〈11〉
USP Octisalate RS


