Nystatin
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
Change to read:
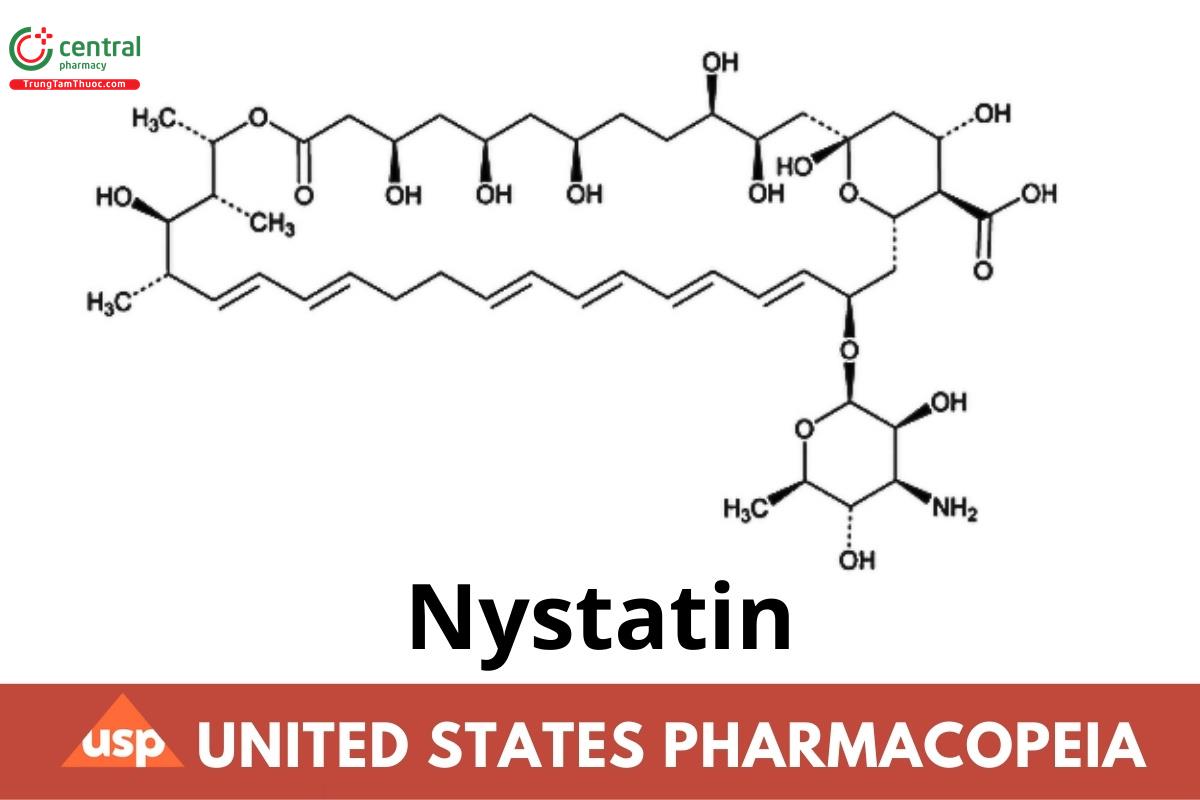
C47H75NO17 926.11 (USP 1-Dec-2022)
Nystatin A;
14,39-Dioxabicyclo[33.3.1]nonatriaconta-19,21,25,27,29,31-hexaene-36-carboxylic acid,
33-[(3-amino-3,6-dideoxy-β-d-mannopyranosyl)oxy]-1,3,4,7,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo,
(1S,3R,4R,7R,9R,11R,15S,16R,17R,18S,19E,21E,25E,27E,29E,31E,33R,35S,36R,37S)-;
(1S,3R,4R,7R,9R,11R,15S,16R,17R,18S,19E,21E,25E,27E,29E,31E,33R,35S,36R,37S)-33-[(3-Amino-3,6-dideoxy-β-d-mannopyranosyl)oxy]-1,3,4,7,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,25,27,29,31-hexaene-36-carboxylic acid
CAS RN®: 1400-61-9; UNII: BDF1O1C72E
1 DEFINITION
Nystatin is a substance or a mixture of two or more substances produced by the growth of Streptomyces noursei Brown et al. (Family Streptomycetaceae). It has a potency of NLT 4400 USP Nystatin Units/mg or, where intended for use in the extemporaneous preparation of oral suspensions, NLT 5000 USP Nystatin Units/mg.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (USP 1-Dec-2022)
Add the following:
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the test for Composition. (USP 1-Dec-2022)
3 ASSAY
3.1 Procedure
(See Antibiotics—Microbial Assays 〈81〉.)
Analysis: Proceed as directed in the chapter.
Acceptance criteria:
NLT 4400 USP Nystatin Units/mg; where intended for use in the extemporaneous preparation of oral suspensions, NLT 5000 USP Nystatin Units/mg
4 SPECIFIC TESTS
Change to read:
Suspendability (where packaged for use in the extemporaneous preparation of oral suspensions)
Analysis
Transfer about 200 mg ▲of nystatin▲ (USP 1-Dec-2022), accurately weighed, to a 250-mL beaker containing 200.0 mL of water, and disperse by stirring gently with a stirring rod. Allow to stand for 2 min, and observe the suspension.
If there is any sediment, assay the undisturbed suspension as directed for Nystatin in Antibiotics—Microbial Assays 〈81〉, using a suitable aliquot blended in a high-speed blender for 3–5 min with a sufficient volume of dimethylformamide to obtain a solution having a concentration of 400 USP Nystatin Units/mL. Dilute this stock solution quantitatively with Buffer B.6 to obtain a test dilution having a nystatin concentration assumed to be equal to the median level of the standard.
Acceptance criteria
The material is in suspension, and little or no sediment is present on the bottom of the beaker.
If there is any sediment, the undisturbed suspension contains NLT 90.0% of the expected number of USP Nystatin Units, based on the potency obtained in the Assay.
Crystallinity 〈695〉 (where packaged for use in the extemporaneous preparation of oral suspensions): Meets the requirements
pH 〈791〉
Sample: 3% aqueous suspension
Acceptance criteria: 6.0–8.0
Loss on Drying 〈731〉
Sample: 100 mg
Analysis: Dry the Sample in a capillary-stoppered bottle under vacuum at a pressure not exceeding 5 mm of mercury at 60° for 3 h.
Acceptance criteria: NMT 5.0%
Composition
Solution A: Acetonitrile and 0.05 M ammonium acetate (29:71)
Solution B: Acetonitrile and 0.05 M ammonium acetate (60:40)
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 25 | 100 | 0 |
| 35 | 0 | 100 |
| 40 | 0 | 100 |
| 45 | 100 | 0 |
| 50 | 100 | 0 |
System suitability solution: Dissolve 20 mg of Nystatin in 25 mL of methanol, and dilute with water to 50 mL. To 10.0 mL of the resulting solution add 2.0 mL of dilute hydrochloric acid, and allow to stand at room temperature for 1 h.
Standard solution: 0.4 mg/mL of USP Nystatin RS in Dimethyl sulfoxide. Protect this solution from light, store refrigerated, and use within 24 h.
Sample solution: 0.4 mg/mL of Nystatin in dimethyl sulfoxide. Protect this solution from light, store refrigerated, and use within 24 h.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 304 nm
Column: 4.6-mm × 15-cm; base-deactivated, end-capped 5-µm packing L1
Column temperature: 30°
Flow rate: 1 mL/min
Injection volume: 20 µL
System suitability
Samples: System suitability solution and Standard solution
[Note—The nystatin A1 peak elutes at 14 min. Identify this peak using the Standard solution.]
Suitability requirements
Resolution: NLT 3.5 between the two major peaks, System suitability solution
Analysis
Sample: Sample solution
Calculate the percentage of each peak:
Result = (rU/rT) × 100
rU = area of each individual peak
rT = total area of all peaks except those eluting in less than 2 min
Acceptance criteria: NLT 85.0% of nystatin A1; NMT 4.0% of any other individual component. Disregard any peaks eluting in less than 2 min.
5 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers.
Labeling: Where packaged for use in the extemporaneous preparation of oral suspensions, the label so states.
USP Reference Standards 〈11〉
USP Nystatin RS


