Norflurane
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
CH2FCF3 102.03
(1,1,1,2-Tetrafluoroethane) CAS RN®: 811-97-2.
1 DEFINITION
Norflurane contains NLT 99.8% of 1,1,1,2-Tetrafluoroethane (CH2FCF3).
This monograph applies to Norflurane synthesized via the synthetic route based on the hydrofluorination of trichloroethylene. This monograph may not be applicable to other synthetic routes, e.g., the route starting from perchloroethylene.
Norflurane is also known as 1,1,1,2-Tetrafluoroethane or HFA-134a.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: Use a 10-cm gas cell. The IR absorption spectrum of the sample exhibits maxima only at the same wavelengths as those of the Norflurane Identification Standard.1
B. GAS CHROMATOGRAPHY: The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the test for Organic Impurities.
3 IMPURITIES
3.1 LIMIT OF HIGH BOILING MATTER
Sample: 100 mL of norflurane
Analysis: Evaporate the Sample in a Goetz tube. When 85 mL has evaporated, ensure that the capillary tube is cooled to 0" by placing it in an ice/water mixture. Maintain the capillary at this temperature for a further 30 min. Note the volume of the residue to the nearest 0.01 mL..
Acceptance criteria: The residual liquid is NMT 0.01 mL.
3.2 LIMIT OF NONVOLATILE RESIDUE
Sample: 500 g of norflurane
Analysis: Evaporate the Sample in a tared dish on a water bath, and dry at 100°-105° for 1 h.
Acceptance criteria: 0.0005% (5 ppm)
Change to read:
3.3 ORGANIC IMPURITIES
Sample solution: Norflurane
Standard solution mixture1: Would include approximately 10 ppm of HFC-125, 1000 ppm of HFC-134, 20 ppm of HFC-152a, 10 ppm of HCC-40, and 10 ppm of HCFC-133a in HFA-134a
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.25 - mm x 120 - m ID, 1.4-µm film thickness, G53
Temperatures
Injection port: 80°
Detector: 200°
Column: See Table 1.
Table 1
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| −10 | 0 | −10 | 10 |
| −10 | 5 | 50 | 10 |
| 50 | 10 | 80 | 3 |
Linear velocity: 20-24 cm/sec at the GC oven initial temperature (-10°)
Carrier gas: Helium
Injection type: Direct. A vaporized sample is added directly to the column via a gas sample valve.
Injection volume: 50 µL
System suitability
Sample: Norflurane
Suitability requirements
Resolution: >1.5 between HFC-134 and HFC-152a
Analysis
Samples: Sample solution and Standard solution mixture
Calculation
Impurityppm = (ASample/AStandard) x CStandard x (1/F)
Where:
ASample = peak area of degradation product in the Sample solution
AStandard = mean impurity peak area of the Standard solution mixture
CStandard = concentration of impurity standard in the Standard solution mixture, ppm
F = relative response factor (see Table 2)
% Impurity = (Impurityppm/1,000,000) x 100% %
Purity = 100% − Σ% Impurities
Acceptance criteria
Purity: NLT 99.8%
Individual impurities: See Table 2.
Table 2
| ▲Peak Elution Order▲(ERR 1-Dec-2023) | Impurity | Relative Retention Time | Relative Response Factor | Acceptance Criteria, (ppm) |
| 1 | HFC-125 | HFC-125 | 1HFC - 125 | 5 |
| 2 | HFC-143a/HFO-1123 | 0.87 x HFC-134 | 0.728HFC - 152a | 5 |
| 3 | PFC-c318 | 0.89 x HFC-134 | 0.693HCC - 40 | 5 |
| 4 | HFC-245cb/HFO-1234yf | 0.92 x HFC-134 | 0.671HFC - 152a | 5 |
| 5 | HFC-134 | HFC-134 | 1HFC - 134 | 1000 |
| 6 | HFC-152a | HFC-152a | 1HFC - 152a | 20 |
| 7 | HFOS-1132/1234ze(e)/1243zf | 0.76x HCC-40 | 0.727HFC - 152a | 5 |
| 8 | HFO-1336mzz | 0.77 x HCC-40 | 0.707HFC - 152a | 5 |
| 9 | HCFC-22/CFC-217ba | 0.78x HCC-40 | 0.586HFC - 125 | 5 |
| 10 | HFC-263fb | 0.85 x HCC-40 | 1.234HCC - 40 | 5 |
| 11 | HCFC-124/124a | 0.94 x HCC-40 | 1.488HFC - 125 | 5 |
| 12 | HCFO-1131a | 0.98HCC - 40 | 1.149HCC - 40 | 5 |
| 13 | HCC-40 | HCC-40 | 1HCC - 40 | 5 |
| 14 | HCFO-1122 | 0.85 x HCFC-133a | 1.019HCC - 40 | 5 |
| 15 | HCFO-1122a-c | 0.86x HCFC-133a | 1.019HCC - 40 | 5 |
| 16 | HCFC-31 | 0.88 x HCFC-133a | 1.234HFC - 125 | 5 |
| 17 | CFC-114/114a | 0.89 x HCFC-133a | 0.974HFC - 125 | 5 |
| 18 | HCFC-133a | HCFC-133a | 1 x HCFC-133a | 5 |
| 19 | HCFO-1131 | 0.98HCC - 40 | 1.124HCC - 40 | 5 |
| Total unsaturates + 245cb + HFC 143a (sum lines 2,4,7,8,12,14,15,19) | — | — | 5 | |
| Any unspecified impurity | — | — | Limit 5 | |
| Total unspecified impurities | — | 1 x HFC-125 | Limit 50 |
3.4 LIMIT OF NITROGEN AND OXYGEN
Sample solution: Headspace above norflurane liquid
Standard solution mixture2: 1.0% (v/v) nitrogen and 0.5% (v/v) oxygen in helium
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: GC
Detector: Thermal conductivity
Column: Glass or stainless steel, 4 - mm x 2 - m with support S13
Temperatures
Injection port: 50°
Detector: 100°
Column: 80°
Carrier gas velocity: 40 mL/min
Carrier gas: Helium
Injection type: Direct. The cylinder containing the headspace gas is connected to the sample loop. A vacuum is applied to the other end of the loop to encourage the gas to flow through.
Injection volume: 250 µL
System suitability
Sample: Sample solution
Suitability requirements
Resolution: >1.5 between nitrogen and oxygen
Analysis
Samples: Sample solution and Standard solution mixture
Calculation:
% Impurity = (ASample/AStandard)/CStandard
Where:
ASample = peak area of nitrogen or oxygen in the Sample solution
AStandard = mean peak area of nitrogen or oxygen in the Standard solution mixture
CStandard = concentration of nitrogen or oxygen in the Standard solution mixture, % v/v
Acceptance criteria: 1.5% of non-condensable gases (total of oxygen and nitrogen)
Change to read:
3.5 HALIDES
Sample: 50 g of norflurane
Standard: Determine the cell constant against known conductivity standards.
Analysis: See Figure 1. Place the sample cylinder into the cylinder support which is positioned on top of the balance. Connect the sample delivery tube to the cylinder and check that needle valve (A) on the delivery tube is closed. Using a clean 100 mL measuring cylinder, a 100 mL aliquot of deionized water is placed into Dreschel bottle A.
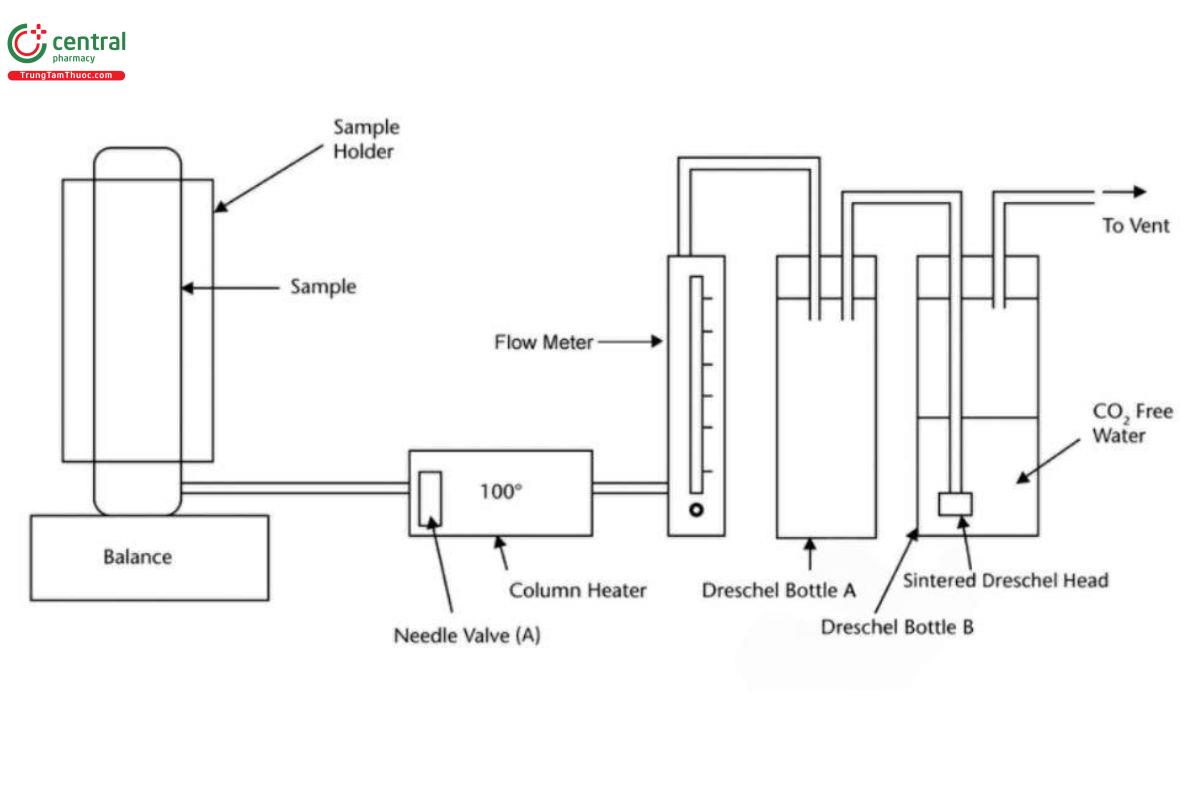
The water is carefully transferred to Dreschel bottle B and finally to a clean and dry beaker. Place the conductivity cell into the sample and slightly agitate the cell in order to remove any air bubbles.
Measure the conductivity of the deionized water in microSiemens/cm after allowing the reading to stabilize and record this value as C1. Pour the deionized water carefully back into Dreschel bottle B and immerse the sintered Dreschel head into it.
Checking that the column heater is in the temperature range of 100 ± 10° note the balance reading (W1) and open the sample cylinder valve.
Open needle valve (A) on the sample delivery tube, and by means of the rotameter, adjust the flow to a steady vaporization rate of 200-300 mL/min.
When approximately 50 g of sample has passed through the Dreschel bottles, the balance reading is noted to the nearest gram (W2) and needle valve (A) on the delivery line is closed immediately, followed by the sample cylinder valve.
The deionized water in Dreschel bottle B is carefully washed into Dreschel bottle A and then into the previously used beaker. The conductivity of the deionized water is re-measured as before (C2).
Calculation
Halides (ppm) = [V x (C2-C1)]/[Ar x (W1-W2)]
Where:
V = volume of deionized water (100 mL)
C = conductivity measure before and after sampling
Ar = atomic weight of fluorine, 19
W = mass of the cylinder before and after sampling
Acceptance criteria: NMT 0.5 ppm
4 RESIDUAL ACIDITY
BROMOCRESOL PURPLE SODIUM SALT TS: Dissolve 0.04 g of bromocresol purple sodium salt in 100 mL of carbon dioxide free water to 100 mL.
Sample: 200 g of norflurane
Analysis: Add 75 mL of carbon dioxide free water and 1 mL of Bromocresol purple sodium salt TS to the three Dreschel bottles (see Figure 2) that have been pre-rinsed with Bromocresol purple sodium salt TS. Add either 0.01 M sodium hydroxide solution or 0.01 M hydrochloric acid solution until the bromocresol purple neutral color develops. Discard the water from Dreschel bottle A. Immerse the sintered Dreschel heads in the water in bottles B and C. Insert the Dreschel head in bottle A.
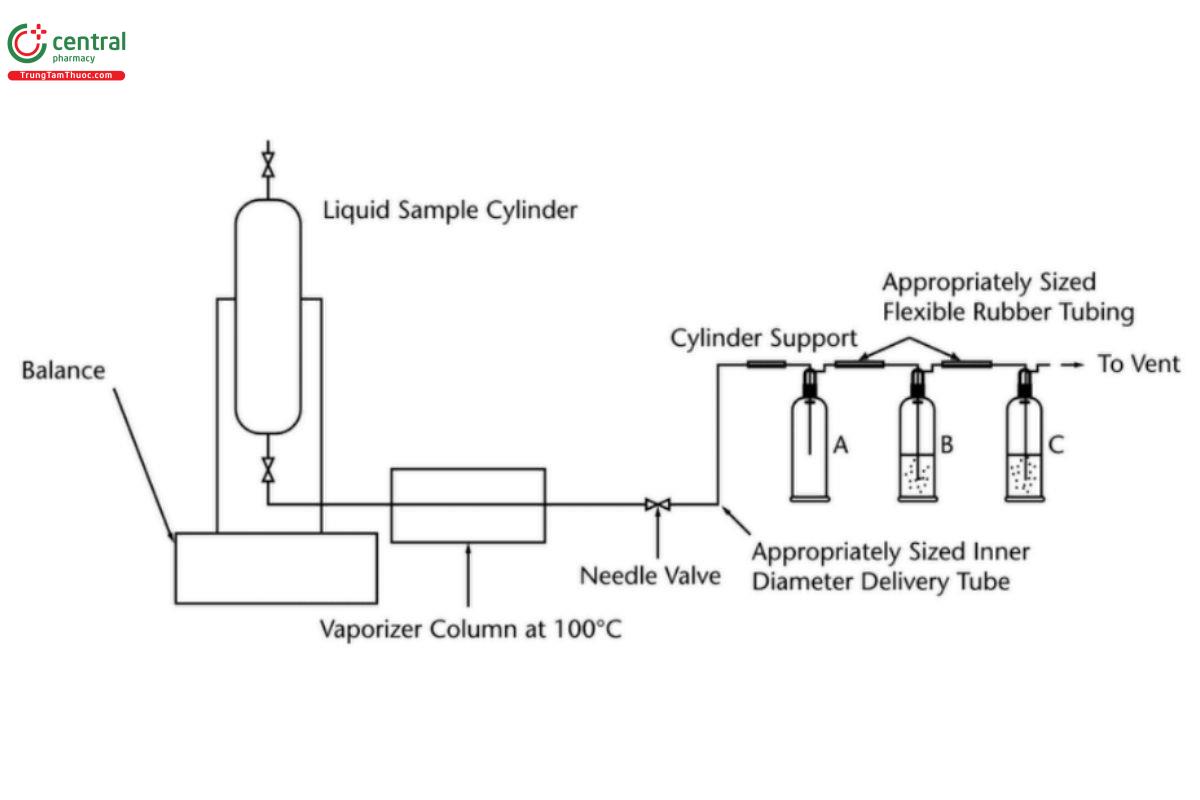
At the initial set up of the apparatus, set the vaporizer temperature to give a reading of 100 ± 10°, using a calibrated digital thermometer. Weigh the sample cylinder to the nearest 1 g (W1), invert the cylinder and position it in the sample cylinder holder. Connect the sample vaporization and delivery tube to the valve outlet.
Open the cylinder valve. Carefully open the needle valve on the sample delivery tube. Adjust the valve so that there is a steady vaporization rate of 150-200 g/h.
When approximately 200 g of sample have been passed through the Dreschel bottles, close the cylinder valve. Close the valve on the sample delivery line and disconnect the sample vaporization and delivery tube. Note the weight of the cylinder to the nearest 1 g (W2).
Pour the water from bottle B into bottle A. If the sample contained acidity then the water in Dreschel bottle B will have turned yellow or will turn yellow upon being added to bottle A. Titrate any acidity with 0.01 M sodium hydroxide solution, using the micro burette, to the same bromocresol purple end point as before.
The water in Dreschel bottle C will remain neutral except in cases of extreme acidity. If it has turned yellow, this solution must be titrated as well and added to that measured in Dreschel bottle B.
Calculation
Total acid (w / w) = (V x 365) / (W1 - W2)
Where:
V = volume (mL) of 0.01 M sodium hydroxide titrated
365 = molecular weight of HCl times 10
Acceptance criteria: ≤0.1 ppm
4.1 WATER DETERMINATION (921)
Norflurane is hygroscopic and as such test preparation is critical to maintaining the residual moisture present in the sample. All apparatus and connection fittings must be dried prior to use. Norflurane must be presented to the coulometric titration vessel as a vaporized liquid. The diagram includes details the apparatus needs to vaporize the sample and present it to the sealed titration cell. Flow rate upon introduction of the sample must be controlled carefully to not only minimize the risk of over-pressurization of the apparatus but also to maximize residence time of the sample vapor in the Karl Fischer titration solution.
Sample: >5 g of Norflurane
Introduce the sample into the titration cell using a system similar to that shown in Figure 3.
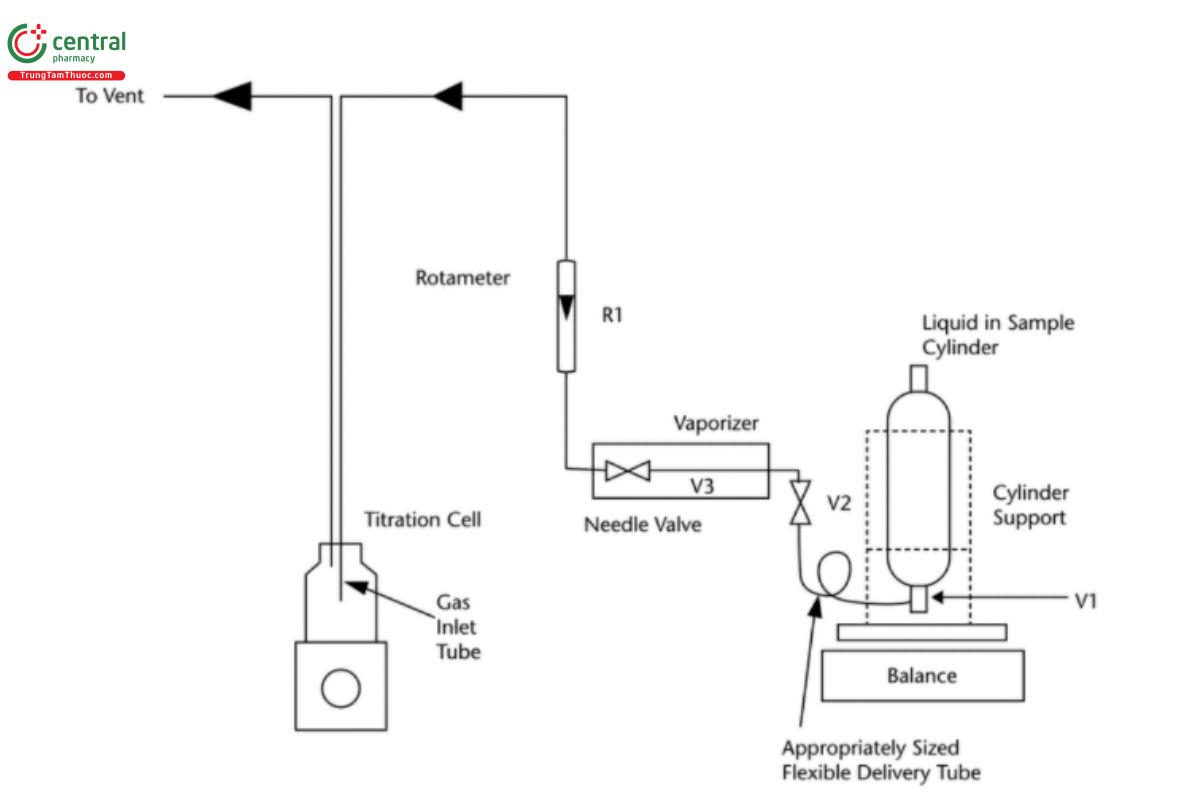
Analysis: Analyze per Water Determination (921), Method J, Method Ic
Acceptance criteria: 10 ppm
5 SPECIFIC TESTS
5.1 APPEARANCE OF SOLUTION
Sample: Transfer 100 mL of norflurane into a Goetz tube and 100 mL of deionized or distilled water into a second Goetz tube. If the outside of the Goetz tube frosts up as it is filled, the ice can be removed by washing with acetone on the outside of the tube.
Analysis: Hold both Goetz tubes against a white surface in good light and view horizontally from the side at 90°. Avoid viewing vertically from the top. Compare the sample against the water for color, clarity, and foreign matter. Next, hold the Goetz tubes against a vertical black background and view horizontally from the side, at 90° to the light source, for turbidity.
Acceptance criteria: Clear and colorless
6 ADDITIONAL REQUIREMENTS
OFFIA PACKAGING AND STORAGE: Preserve in DOT approved gas storage cylinders and store within the temperature range recommended by the manufacturer.
1 Laboratories wishing to purchase these can approach zephexsales@kouraglobal.com and request a quotation for the standard(s) and their shipment.
2 Can be obtained from a commercial gas manufacturer.


