Nizatidine
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
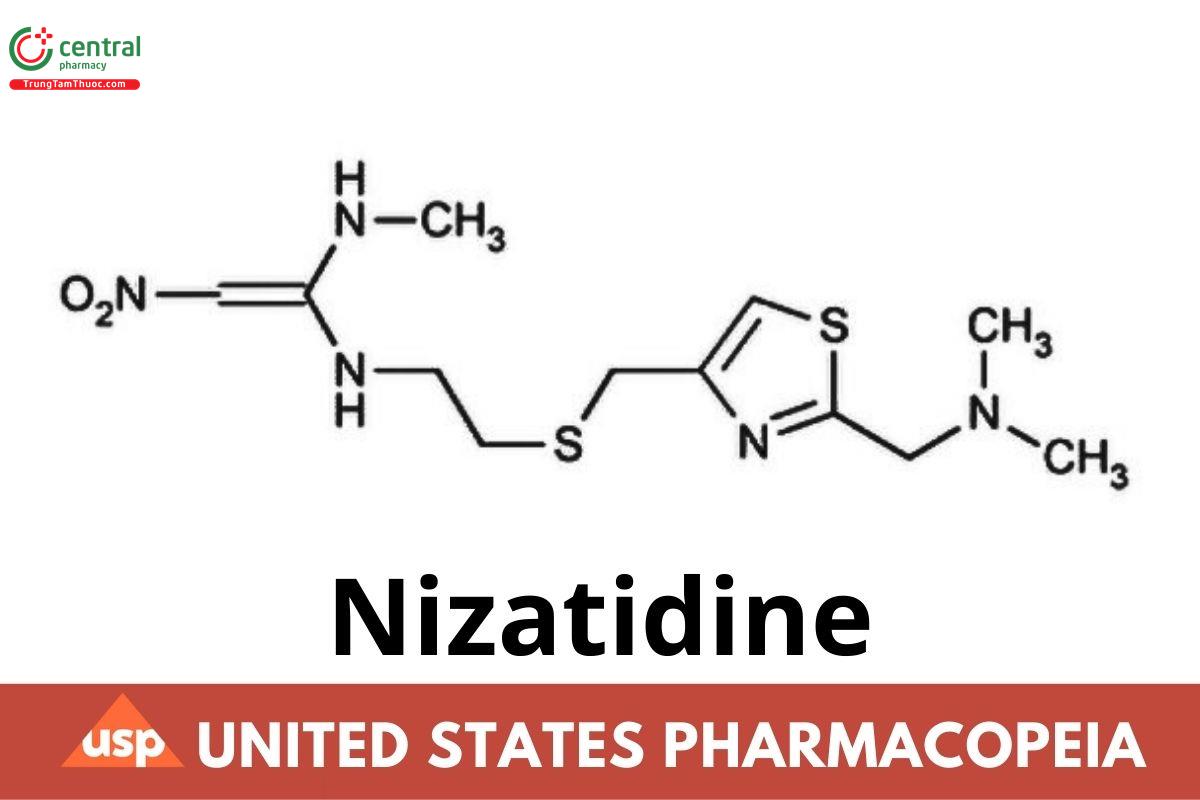
C₁₂H₂₁N₅O₂S₂ 331.46
1,1-Ethenediamine, N-[2-[[[2-[(dimethylamino)methyl]-4-thiazolyl]methyl]thio]ethyl]-N′-methyl-2-nitro-.
N-[2-[[[2-[(Dimethylamino)methyl]-4-thiazolyl]methyl]thio]ethyl]-N′-methyl-2-nitro-1,1-ethenediamine CAS RN®: 76963-41-2; UNII: P41PML4GHR.
Nizatidine contains not less than 98.0 percent and not more than 101.0 percent of C₁₂H₂₁N₅O₂S₂, calculated on the dried basis.
1 Packaging and storage
Preserve in tight, light-resistant containers.
1.1 USP Reference standards 〈11〉
USP Nizatidine RS
2 Identification
Change to read:
A: Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 〈197K〉
B: The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that of the Standard preparation as obtained in the Assay.
Loss on drying 〈731〉 - Dry about 2 g, accurately weighed, at 100° for 1 hour: it loses not more than 1.0% of its weight.
Residue on ignition 〈281〉: Not more than 0.1%.
3 Chromatographic purity
Solution A - Use Buffer solution prepared as directed in the Assay.
Solution B - Use methanol.
Diluent - Prepare a mixture of Solution A and Solution B (76:24).
Mobile phase - Use variable mixtures of Solution A and Solution B as directed for the Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography 〈621〉).
Standard solutions - Dissolve an accurately weighed quantity of USP Nizatidine RS quantitatively, and stepwise if necessary, in Diluent, sonicating if necessary, to obtain a solution having a known concentration of 50 µg per mL (Standard solution 1). Quantitatively dilute portions of Standard solution 1 with Diluent to obtain Standard solution 2 and Standard solution 3 having known concentrations of 25 µg per mL and 15 µg per mL, respectively.
Test solution - Prepare a solution of Nizatidine in Diluent having a concentration of about 5 mg per mL.
Chromatographic system (see Chromatography 〈621〉) - The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. The chromatograph is programmed as follows.
| Time (minutes) | Solution A (%) | Solution B (%) | Elution |
| 0–3 | 76 | 24 | isocratic |
| 3–20 | 76→50 | 24→50 | linear gradient |
| 20–45 | 50 | 50 | isocratic |
| 45–50 | 50→76 | 50→24 | linear gradient |
| 50–70 | 76 | 24 | isocratic |
Make adjustments to the composition of the Mobile phase, if necessary, to obtain a retention time of about 12 minutes for the main nizatidine peak (see System Suitability under Chromatography 〈621〉). Chromatograph Standard solution 1, and record the peak areas as directed for Procedure: the tailing factor is not more than 2.0.
Procedure - Separately inject equal volumes (about 50 µL) of Standard solution 1, Standard solution 2, Standard solution 3, and the Test solution into the chromatograph, and allow the Test solution to elute for not less than three times the retention time of nizatidine. Record the chromatograms, and measure the areas for all the peaks. The sum of the peak areas, excluding the nizatidine peak area, obtained from the Test solution is not more than three times the main peak area obtained from Standard solution 2; and no single peak area obtained from the Test solution is greater than the main peak area obtained from Standard solution 3: not more than 0.3% of any individual impurity is found; and not more than 1.5% of total impurities is found.
4 Assay
Buffer solution - Prepare a 0.1 M solution by dissolving 5.9 g of ammonium acetate in 760 mL of water. Add 1 mL of diethylamine, and adjust with acetic acid to a pH of 7.5.
Mobile phase - Prepare a filtered and degassed mixture of Buffer solution and methanol (76:24). Make adjustments if necessary (see System Suitability under Chromatography 〈621〉).
Standard preparation - Dissolve an accurately weighed quantity of USP Nizatidine RS in Mobile phase, sonicating if necessary, to obtain a solution having a known concentration of about 0.3 mg per mL.
Assay preparation - Transfer an accurately weighed quantity of 15 mg of Nizatidine to a 50-mL volumetric flask, dissolve in Mobile phase, sonicating if necessary, dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography 〈621〉) - The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 15-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. Chromatograph the Standard preparation, and record the peak areas as directed for Procedure: the column efficiency is not less than 1500 theoretical plates; the tailing factor is not more than 2.0; and the relative standard deviation for replicate injections is not more than 1.5%.
Procedure - Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the quantity, in mg, of C₁₂H₂₁N₅O₂S₂ in the portion of Nizatidine taken by the formula:
50C(rᵤ/rₛ)
in which C is the concentration, in mg per mL, of USP Nizatidine RS in the Standard preparation; and rᵤ and rₛ are the peak areas obtained from the Assay preparation and the Standard preparation, respectively.


