Nitrofurantoin Capsules
If you find any inaccurate information, please let us know by providing your feedback here

Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Nitrofurantoin Capsules contain NLT 90.0% and NMT 110.0% of the labeled amount of nitrofurantoin (C8H6N4O5).
2 IDENTIFICATION
A. INFRARED ABSORPTION
Sample: Add 10 ml of 6 N acetic acid to a quantity of the contents of Capsules equivalent to 100 mg of nitrofurantoin. Boil the solution for a few min, and filter while hot. Cool to room temperature, collect the precipitate of nitrofurantoin, and dry at 105° for 1 h.
Acceptance criteria: The IR absorption spectrum of a Mineral oil dispersion of the precipitate so obtained exhibits maxima only at the same wavelength as that of a similar solution of USP Nitrofurantoin RS.
B. The retention time of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Solution A: Dissolve 6.8 g of monobasic potassium phosphate in 500 mL of water. Add a volume of 1.0 N sodium hydroxide (about 30 mL) sufficient to adjust to a pH of 7.0, and dilute with water to 1 L.
Mobile phase: Acetonitrile and Solution A (3:22)
Internal standard solution: 1 mg/mL of acetanilide in water
Standard solution: Dissolve 50 mg of USP Nitrofurantoin RS in 40.0 mL of dimethylformamide, and add 50.0 mL of Internal standard solution.
Sample solution: Transfer, as completely as possible, the contents of 20 Capsules to a 500-mL flask. Place the emptied Capsules in a beaker, add 25 mL of dimethylformamide, and agitate for 1 min. Decant into the flask containing the Capsule contents. Rinse the emptied Capsules with another two 25-mL portions of dimethylformamide, and decant into the flask. Add sufficient dimethylformamide to bring the volume to about 250 mL. Insert the stopper in the flask, and shake by mechanical means for 15 min. Dilute with dimethylformamide to volume, and mix. If necessary, the sample may be homogenized using a disperser. Pass through a medium-porosity, sintered-glass filter into a suitable flask. Transfer an aliquot, equivalent to 50 mg of nitrofurantoin, to a flask. Add an accurately measured volume of dimethylformamide to bring the volume in the flask to 40.0 mL. To the flask add 50.0 mL of Internal standard solution, mix, and cool to room temperature. Pass a portion of the solution through a nylon filter of 0.45-µm pore size, discarding the first few mL of the filtrate.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 3.9-mm x 30-cm; packing L1
Injection volume: 5-10 µL
System suitability
Sample: Standard solution
[NOTE-Adjust the operating parameters so that the retention time of the nitrofurantoin peak is about 8 min, and the peak heights are about half full-scale.]
Suitability requirements
Resolution: NLT 3.0 between acetanilide and nitrofurantoin
Relative standard deviation: NMT 2.0%, determined from peak response ratios of replicate injections
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of nitrofurantoin (C8H6N4O5) in the portion of the powder included in the sample aliquot:
Result = (RU/RS) × (CS/CU)
RU = peak response ratio from the Sample solution
RS = peak response ratio from the Standard solution
CS = concentration of USP Nitrofurantoin RS in the Standard solution (mg/mL)
CU = nominal concentration of the Sample solution (mg/mL)
Acceptance criteria: 90.0%-110.0%
4 PERFORMANCE TESTS
Change to read:
4.1 DISSOLUTION (711)
4.1.1 Test 1 (where it is labeled as containing nitrofurantoin macrocrystals)
Medium: pH 7.2 (±0.05) phosphate buffer; 900 mL
Apparatus 1: 100 rpm
Times: 1, 3, and 8 h
Standard solution: USP Nitrofurantoin RS in Medium
Sample solution: Pass a portion of the solution under test through a suitable filter. Dilute with Medium, if necessary.
Blank: Medium
Instrumental conditions
Mode: UV
Analytical wavelength: 375 nm
Tolerances: See Table 1.
Table 1
| Time (h) | Amount Dissolved |
| 1 | 20%–60% |
| 3 | NLT 45% |
| 8 | NLT 60% |
The percentage of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved at the 1-h point conforms to Dissolution (711), Acceptance Table 2 and the percentages dissolved at the 3- and 8-h points conform to the criteria for the final test time in Dissolution (711), Acceptance Table 2.
4.1.2 Test 2 (where it is labeled as containing both nitrofurantoin macrocrystalline and monohydrate forms): If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 2
Acid medium: 0.01 N hydrochloric acid for 1 h; 900 mL
pH 7.5 buffer medium: Prepare a pH 7.5 buffer concentrate by dissolving 62.2 g of potassium hydroxide and 129.3 g of monobasic potassium phosphate in water, dilute with water to 1 L, and mix. After 1 h, change the Acid medium to pH 7.5 buffer medium by adding 50 mL of pH 7.5 buffer concentrate, and run for an additional 6 h.
Apparatus 2: 100 rpm, with sinkers made of Teflon-coated steel wire prepared by forming a coil approximately 22 mm long from a 13-cm length of 20-gauge wire (see Figure 1)
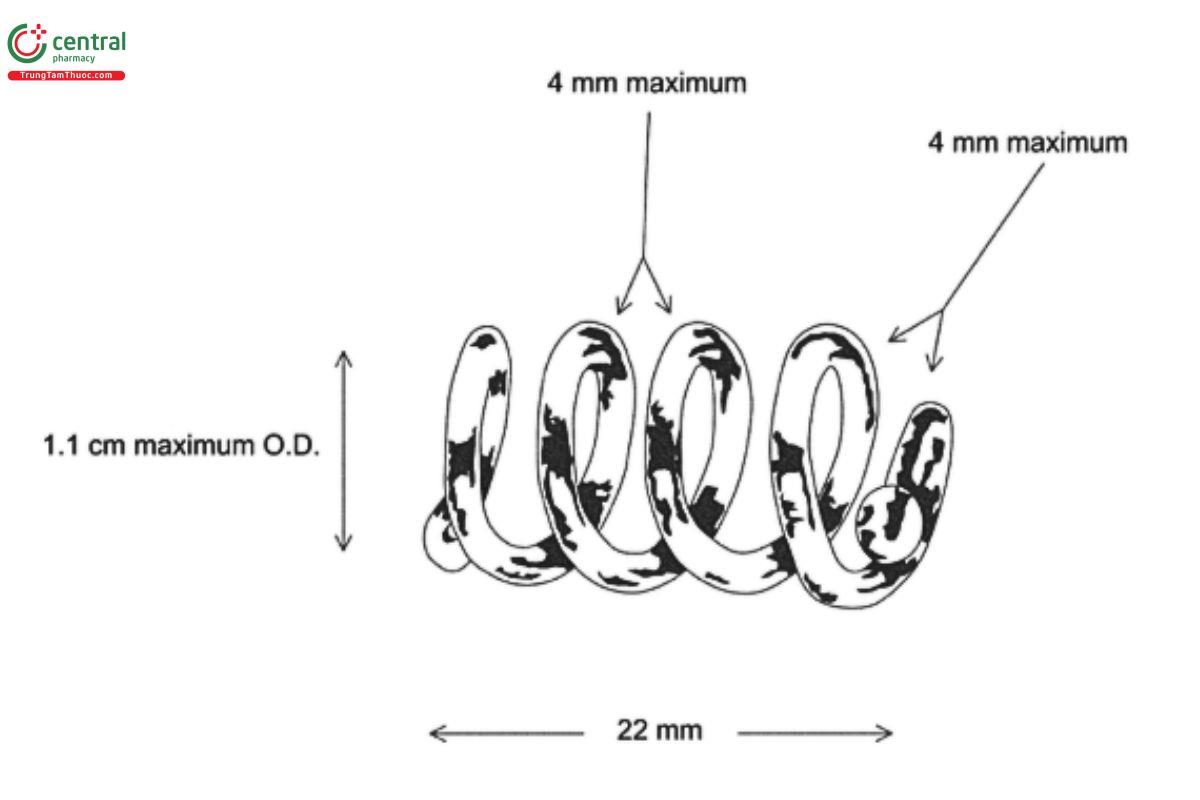
Times: 1, 3, and 7 h
Acid-stage standard solution: 0.025 mg/mL of USP Nitrofurantoin RS in Acid medium
Buffer-stage standard solution: 0.075 mg/mL of USP Nitrofurantoin RS in pH 7.5 buffer medium
Instrumental conditions
Mode: UV
Analytical wavelength: 375 nm
Analysis: Calculate the percentages of the labeled amount (Qi) of nitrofurantoin (C8H6N4O5) dissolved from UV absorbances at the isosbestic wavelength at about 375 nm on filtered portions of each solution under test, suitably diluted, if necessary, with Acid medium or pH 7.5 buffer medium when appropriate, in comparison with the appropriate Standard solution.
Tolerances: See Table 2.
Table 2
| Time (h) | Amount Dissolved (Individual) | Amount Dissolved (Mean) |
| 1 | 2%-16% | 5%–13% |
| 3 | 27%-69% | 39%–56% |
| 7 | NLT 68% | NLT 81% |
The percentages of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved at the specified times conform to Table 3.
Table 3
| Level | Number Tested | Criteria |
| L1 | 12 | The mean percentage of dissolved label claim lies within the range for the means at each interval and is NLT the stated amount at the final test time. All individual values lie within the ranges for the individuals at each interval and are NLT the stated amount at the final test time. |
| L2 | 12 | The mean percentage of dissolved label claim lies within the range for the means at each interval and is NLT the stated amount at the final test time. NMT 2 of the 24 individual values lie outside the stated ranges for individuals at each interval, and NMT 2 of 24 are less than the stated amount at the final test time. |
4.1.3 Test 3 (where it is labeled as containing both nitrofurantoin macrocrystalline and monohydrate forms): If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 3
Acid medium, pH 7.5 buffer medium, Apparatus 2, Times, Acid-stage standard solution, Buffer-stage standard solution, and Analysis: Proceed as directed in Test 2.
Tolerances: See Table 4.
Table 4
| Time (h) | Amount Dissolved (Individual) | Amount Dissolved (Mean) |
| 1 | 2%-16% | 5%–13% |
| 3 | 50%-80% | 55%–75% |
| 7 | NLT 85% | NLT 90% |
The percentages of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved at the specified times conform to Dissolution (711). Acceptance Table 2.
4.1.4 Test 4 (where it is labeled as containing both nitrofurantoin macrocrystalline and monohydrate forms): If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 4
Acid medium: 0.01 N hydrochloric acid for 1 h; 900 mL, deaerated
pH 7.5 buffer medium: Prepare a pH 7.5 buffer concentrate by dissolving 62.2 g of potassium hydroxide and 129.3 g of monobasic potassium phosphate in water, dilute with water to 1 L, and mix. After 1 h change the Acid medium to pH 7.5 buffer medium by adding 50 mL of pH 7.5 buffer concentrate, and run for an additional 9 h.
Apparatus 2: 100 rpm, with helix sinkers
Times: 1, 3, and 10 h
Standard stock solution: Transfer 25 mg of USP Nitrofurantoin RS to a 10-mL volumetric flask. Add 7.5 mL of dimethylformamide, and sonicate until dissolved. Allow to cool to room temperature, and dilute with dimethylformamide to volume.
Acid-stage standard solution: Dilute 2.0 mL of the Standard stock solution with Acid medium to 200 mL.
Buffer-stage standard solution: Transfer 3.0 mL of the Standard stock solution to a 100-mL volumetric flask, and dilute with pH 7.5 buffer medium to volume.
Stock capsule shell blank: Place 10 empty, clean Capsules into a 900-mL volumetric flask, and add 800 mL of Acid medium. Gently heat to 37 ± 0.5°, and stir until all the Capsules are dissolved. Allow to cool to room temperature, and dilute with Acid medium to volume.
Buffer-stage capsule shell blank: Transfer 100.0 mL of the Stock capsule shell blank to a 1000-mL volumetric flask. Add 56 mL of pH 7.5 buffer medium, dilute with Acid medium to volume, and mix. Filter, using the same filter as for the Sample solution.
Sample solution: Pass portions of the solution under test through a 1.2-µm glass/0.45-µm polyethersulfone combination filter, discarding the first few mL.
Instrumental conditions
Mode: UV
Analytical wavelength: 375 nm
Analysis: Calculate the percentages of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved from portions of the Sample solution in comparison with the appropriate Acid-stage standard solution or Buffer-stage standard solution. Correct for the appropriate capsule shell blank absorbance, using a 0.1-cm cell, and the appropriate medium as the blank.
Tolerances: See Table 5.
Table 5
| Time (h) | Amount Dissolved |
| 1 | NMT 25% |
| 3 | 25%–50% |
| 10 | NLT 80% |
The percentages of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved at the specified times conform to Dissolution (711), Acceptance Table 2.
4.1.5 Test 5 (where it is labeled as containing both nitrofurantoin macrocrystalline and monohydrate forms): If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 5
Acid medium: 0.01 N hydrochloric acid for 1 h; 900 mL, deaerated
Buffer concentrate: 60 g/L of potassium hydroxide and 129.3 g/L of monobasic potassium phosphate in water
pH 7.5 buffer medium: Prepare by adding 60 mL of Buffer concentrate to 890 mL of Acid medium.
Apparatus 2: 100 rpm, with Teflon-coated sinkers and paddles
Times: 1, 3, and 7 h
Standard stock solution: 2.48 mg/mL of USP Nitrofurantoin RS in acetonitrile. Sonicate using 50% of the final volume of acetonitrile to dissolve. Use an amber volumetric flask.
Acid-stage standard solution: 24.8 µg/mL of USP Nitrofurantoin RS in Acid medium from Standard stock solution. Use an amber volumetric flask.
Buffer-stage standard solution: 74.4 µg/mL of USP Nitrofurantoin RS in pH 7.5 buffer medium from Standard stock solution. Use an amber volumetric flask.
Acid-stage sample solution: After 1 h, collect 10 mL of the solution under test, and pass through a 0.45-µm PVDF filter, discarding the first 5 mL of the filtrate.
Buffer-stage sample solution: After removing 10 ml. of Acid medium, add 60 mL of pH 7.5 buffer medium. The pH of the resulting medium should be about 7.5. Continue the dissolution for another 2 h and 6 h. Collect 10 ml. at each time point, and pass through a 0.45-µm PVDF filter, discarding the first 5 mL of the filtrate.
Acid-stage blank: Use Acid medium.
Buffer-stage blank: Use pH 7.5 buffer medium.
Instrumental conditions
Mode: UV
Analytical wavelength: 375 nm.
Cell: 0.5 cm for acid-stage and 0.1 cm cm for buffer-stage
Analysis
Samples: Acid-stage standard solution, Buffer-stage standard solution, Acid-stage sample solution, Buffer-stage sample solution, Acid-stage blank, and Buffer-stage blank
Calculate the concentration (C) of nitrofurantoin (C8H6N4O5) in the sample withdrawn from the vessel at each time point (i):
Result = (AU/AS) × CS
AU = absorbance of the Sample solution
AS = absorbance of the Standard solution
CS = concentration of the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved at each time point (1):
Result1 = C1 x V1 x (1/L) x 100
Result2 = [(C2 x V2)+ (C1 x VS)] x (1/L) x 100
Result3 = {(C3 x V3) + [(C2 + C1) x VS]} x (1/L) x 100
Ci = concentration of nitrofurantoin in the portion of sample withdrawn at the specified time point (mg/mL)
V1 = volume of medium, 900 mL
L = label claim (mg/Capsule)
V2 = volume of medium, 950 mL
VS = volume of the Sample solution withdrawn at each time point, 10 mL
V3 = volume of medium, 940 mL
Tolerances: See Table 6.
Table 6
| Time Point (i) | Time (h) | Amount Dissolved (Individual) | Amount Dissolved (Mean) |
| 1 | 1 | NMT 12% | NMT 12% |
| 2 | 3 | NLT 80% | 80%–100% |
| 3 | 7 | NLT 85% | NLT 90% |
The percentages of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved at the specified times conform to Table 7.
Table 7
| Level | Number Tested | Criteria |
| L1 | 12 | The mean percentage of dissolved label claim lies within the range for the means at each interval and is NLT the stated amount at the final test time. All individual values lie within the ranges for the individuals at each interval and are NLT the stated amount at the final test. time. |
| L2 | 12 | If the requirements of level L1 are not met, test another twelve (12) Capsules. The requirements are met if the mean percentage of dissolved label claim of all 24 Capsules tested lies within the range for the means at each interval and is NLT the stated amount at the final test time. NMT 2 of the 24 individual values of Capsules lie outside the stated range for individuals at each interval, and NMT 2 of 24 Capsules are less than the stated amount at the final test time. |
4.1.6 Test 6 (where it is labeled as containing both nitrofurantoin macrocrystalline and monohydrate forms): If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 6
Acid medium: 0.01 N hydrochloric acid; 900 mL
pH 7.5 buffer concentrate: Prepare a pH 7.5 buffer concentrate by dissolving 62.2 g of potassium hydroxide and 129.3 g of monobasic potassium phosphate in water and dilute with water to 1 L.
pH 7.5 buffer medium: 900 mL of Acid medium and 50 mL of pH 7.5 buffer concentrate
Apparatus 2: 100 rpm, with sinkers made of Teflon-coated steel wire prepared by forming a coil approximately 22 mm long from a 13-cm length of 20-gauge wire (see Eigure 1 in Dissolution Test 2)
Times
Acid stage: 1 h
Buffer stage: 3, 4, and 7 h
Acid-stage standard stock solution: 0.11 mg/mL of USP Nitrofurantoin RS in Acid medium prepared as follows. Weigh a suitable amount of USP Nitrofurantoin RS in a volumetric flask and add about 2.5% of the flask volume of N.N-dimethylformamide, Sonicate to dissolve completely. Dilute with Acid medium to final volume.
Acid-stage standard solution: 4.4 µg/mL of USP Nitrofurantoin RS in Acid medium from Acid-stage standard stock solution
Buffer-stage standard stock solution: 0.11 mg/mL of USP Nitrofurantoin RS in pH 7.5 buffer medium prepared as follows. Weigh a suitable amount of USP Nitrofurantoin RS in a volumetric flask and add about 2.5% of the flask volume of N.N-dimethylformamide. Sonicate to dissolve completely. Dilute with pH 7.5 buffer medium to final volume.
Buffer-stage standard solution: 4.4 µg/mL of USP Nitrofurantoin RS in pH 7.5 buffer medium from Buffer-stage standard stock solution
Acid-stage sample solution: Pass portions of the solution under test through a suitable filter and discard the first few mL. Dilute with Acid medium, if necessary.
Buffer-stage sample solution: Pass portions of the solution under test through a suitable filter and discard the first few mL. Dilute with pH 7.5 buffer medium, if necessary.
Instrumental conditions
Mode: UV
Analytical wavelength: 375 nm
Dissolution medium: After 1 h in the Acid medium, withdraw 10 mL of the solution under test and add 50 ml. of pH 7.5 buffer concentrate.
Analysis: After 1 h in Acid medium, withdraw 10 mL of solution under test. Add 10 mL of Acid medium, previously warmed to 37 ± 0.5°. Add 50 mL of pH 7.5 buffer concentrate, previously warmed to 37 ± 0.5° and continue the test for 6 h more. At specified times, withdraw 10 mL of solution under test and replace with 10 mL of pH 7.5 buffer medium, previously warmed to 37 ± 0.5°.
Calculate the percentages of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved from portions of the Acid-stage sample solution or Buffer-stage sample solution in comparison with the appropriate Acid-stage standard solution or Buffer-stage standard solution.
Correct for the appropriate capsule shell blank absorbance and the appropriate medium as the blank.
Tolerances: See Table 8.
Table 8
| Time (h) | Amount Dissolved (Individual) | Amount Dissolved (Mean) |
| 1 | 2%–16% | 3%–11% |
| 3 | 15%–45% | 22%–37% |
| 4 | 45%–95% | 65%–85% |
| 7 | NLT 80% | NLT 85% |
The percentages of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved at the specified times conform to Dissolution (711), Acceptance Table 2.
4.1.7 Test 7 (where it is labeled as containing both nitrofurantoin macrocrystalline and monohydrate forms): If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 7
Acid medium: 0.01 N hydrochloric acid, degassed; 900 mL
Buffer concentrate: 62.2 g/L of potassium hydroxide and 129.3 g/L of monobasic potassium phosphate in water
pH 7.5 buffer medium: Prepare by adding 50 mL of Buffer concentrate to 900 mL of Acid medium. Adjust to pH 7.5 ± 0.05 with 1 N hydrochloric acid or 1 N potassium hydroxide
Apparatus 2: 100 rpm, with Teflon-coated helix sinkers
Times: 1, 3, and 7 h
Standard stock solution: 2.5 mg/mL of USP Nitrofurantoin RS in dimethylformamide. Sonicate to dissolve prior to final dilution.
Acid stage standard solution: 0.025 mg/mL of USP Nitrofurantoin RS in Acid medium from Standard stock solution. Prepare this solution immediately before use by diluting from the Standard stock solution.
Buffer stage standard solution: 0.075 mg/mL of USP Nitrofurantoin RS in pH 7.5 buffer medium from Standard stock solution.
Acid stage sample solution: After 1 h, collect 10 mL of the solution under test, and pass through a suitable filter of 0.45-µm pore size, transferring the first 5 mL of the filtrate back into the dissolution vessel.
Buffer stage sample solution: After removing 10 mL of Acid medium, add 50 mL of pH 7.5 buffer medium. Adjust the pH of the resulting medium to 7.5 ± 0.05 with 1 N hydrochloric acid or 1 N potassium hydroxide, if necessary. Continue collecting 10-mL solution under test at each time point, and pass through a suitable filter of 0.45-um pore size, transferring the first 5 mL of the filtrate back into the dissolution vessel.
Acid stage blank: Use Acid medium.
Buffer stage blank: Use pH 7.5 buffer medium.
Instrumental conditions
Mode: UV
Analytical wavelength: 375 nm
Cell: 0.5 cm for acid stage and 0.1 cm for buffer stage
System suitability
Sample: Acid stage standard solution
Suitability requirement
Relative standard deviation: NMT 2.0%
Analysis
Samples: Acid stage standard solution, Buffer stage standard solution, Acid stage sample solution, Buffer stage sample solution, Acid stage blank, and Buffer stage blank
Calculate the concentration (Ci) of nitrofurantoin (C8H6N4O5) in the sample withdrawn from the vessel at each time point (i):
Result = (AU/AS) × CS
AU = absorbance of the Sample solution A
AS = absorbance of the Standard solution
CS = concentration of the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved at each time point (1):
Result1 = C1 x V1 x (1/L) x 100
Result2 = [(C2 x V2)+ (C1 x VS)] x (1/L) x 100
Result3 = {(C3 x V3) + [(C2 + C1) x VS]} x (1/L) x 100
Ci = concentration of nitrofurantoin in the portion of sample withdrawn at the specified time point (mg/mL)
V1= volume of medium, 900 mL.
L = label claim (mg/Capsule)
V2 = volume of medium, 945 mL
VS = volume of the Sample solution withdrawn at each time point, 5 mL
V3 = volume of medium, 940 mL
Tolerances: See Table 9.
Table 9
| Time point (i) | Time (h) | Amount Dissolved (%) |
| 1 | 1 | NMT 23 |
| 2 | 3 | 55–85 |
| 3 | 7 | NLT 85 |
The percentages of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved at the specified times conform to Dissolution (711), Acceptance Table 2.
4.1.8 Test 8 (where it is labeled as containing both nitrofurantoin macrocrystalline and monohydrate forms): If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 8
Acid medium, pH 7.5 buffer medium, Apparatus 2, Times, Acid-stage standard solution, Buffer-stage standard solution, Instrumental conditions, and Analysis: Proceed as directed in Dissolution Test 2.
Tolerances: See Table 10.
Table 10
| Time (h) | Amount Dissolved (individual, %) | Amount Dissolved (mean, %) |
| 1 | NMT 17 | NMT 17 |
| 3 | 27–69 | 35–61 |
| 7 | NLT 68 | NLT 80 |
The percentages of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved at the specified times conform to Table 11.
Table 11
| Level | Number Tested | Criteria |
| L1 | 12 | The mean percentage of dissolved label claim lies within the range for the means at each interval and is NLT the stated amount at the final test time. All individual values lie within the ranges for the individuals at each interval and are NLT the stated amount at the final test time. |
| L2 | 12 | The mean percentage of dissolved label claim lies within the range for the means at each interval and is NLT the stated amount at the final test time. NMT 2 of the 24 individual values lie outside the stated ranges for individuals at each interval, and NMT 2 of 24 are less than the stated amount at the final test time. |
4.1.9 Test 9 (where it is labeled as containing nitrofurantoin macrocrystals): If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 9
Protect solutions containing nitrofurantoin from light.
Medium: 0.05 M pH 7.2 phosphate buffer (6.8 g/L of potassium phosphate monobasic in water. Adjust with 10% (w/v) sodium hydroxide solution to a pH of 7.2.); 900 mL
Apparatus 1: 100 rpm
Times
For 25-mg strength: 0.5, 1, and 6 h
For 50-mg strength: 1, 3, and 8h
For 100-mg strength: 1, 3, and 12 h
Standard stock solution: 1.1 mg/mL of USP Nitrofurantoin RS prepared as follows. Dissolve a suitable amount of USP Nitrofurantoin RS in about 20% of the flask volume of dimethylformamide. Dilute with Medium to volume.
Standard solution: (L/900) mg/mL of USP Nitrofurantoin RS from Standard stock solution in Medium, where L is the label claim in mg/Capsule
Sample solution: At the times specified, withdraw 10 mL of the solution under test and replace with 10 mL of fresh Medium. Centrifuge a portion of the solution withdrawn and use the supernatant. [NOTE-Centrifuge at 5000 rpm for about 5 min may be suitable.]
Instrumental conditions
Mode: UV
Analytical wavelength: 375 nm
Cell
For 25-mg strength: 0.2 cm
For 50-and 100-mg strength: 0.1 cm
Blank: Medium
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (Ci) of nitrofurantoin (C8H6N4O5) in the sample withdrawn from the vessel at each time point (i):
Result = (AU/AS) × CS
AU = absorbance of the Sample solution
AS = absorbance of the Standard solution A
CS = concentration of USP Nitrofurantoin RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved at each time point (i):
Result1 = C1 x V x (1/L) x 100
Result2 = [(C2 x V)+ (C1 x VS)] x (1/L) x 100
Result3 = {(C3 x V) + [(C2 + C1) x VS]} x (1/L) x 100
Ci = concentration of nitrofurantoin in the portion of sample withdrawn at time point i (mg/mL)
V = volume of the Medium, 900 mL
L = label claim (mg/Capsule)
VS = volume of the Sample solution withdrawn at each time point and replaced with Medium, 10 mL
Tolerances
For 25-mg strength: See Table 12.
For 50-mg strength: See Table 13.
For 100-mg strength: See Table 14.
Table 12
| Time point (i) | Time (h) | Amount Dissolved (%) |
| 1 | 0.5 | 15–35 |
| 2 | 1 | 35–55 |
| 3 | 6 | NLT 80 |
Table 13
| Time point (i) | Time (h) | Amount Dissolved (%) |
| 1 | 1 | 29–49 |
| 2 | 3 | 55–75 |
| 3 | 8 | NLT 80 |
Table 14
| Time point (i) | Time (h) | Amount Dissolved (%) |
| 1 | 1 | 27–47 |
| 2 | 3 | 48–68 |
| 3 | 12 | NLT 80 |
The percentages of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved at the times specified conform to Dissolution (711), Acceptance Table 2.
4.1.10 Test 10 (where it is labeled as containing both nitrofurantoin macrocrystalline and monohydrate forms): If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 10
Acid stage medium: 0.01 N hydrochloric acid: 900 mL
Solution A: 62.2 g/L of potassium hydroxide and 129.3 g/L of monobasic potassium phosphate in water
Buffer stage medium: Add 50 ml. of Solution A to 850 mL of Acid stage medium. The pH of this solution is 7.5.
Apparatus 2: 100 rpm, with sinkers made of Teflon-coated steel wire prepared by forming a coil approximately 22 mm long from a 13-cm length of 20-gauge wire
Times
Acid stage: 1 h
Buffer stage: 3 and 7 h. The times in the Buffer stage medium include the time in the Acid stage medium.
Standard stock solution: 0.5 mg/mL of USP Nitrofurantoin RS in dimethylformamide. Sonicate to dissolve.
Acid stage standard solution: 0.01 mg/mL of USP Nitrofurantoin RS from the Standard stock solution in Acid stage medium
Buffer stage standard solution: 0.01 mg/mL of USP Nitrofurantoin RS from the Standard stock solution in Buffer stage medium
Acid stage sample solution: After 1 h, withdraw 50 mL of the solution under test, and pass through a suitable filter of 0.45-µm pore size, discarding the first 4 mL of the filtrate. Dilute with Acid stage medium to a concentration similar to that of the Acid stage standard solution.
Buffer stage sample solution: Add 50 mL of Solution A to the remaining Acid stage medium in the vessel and continue the test. At the specified time points, withdraw a suitable volume of the solution under test and replace with the same volume of Buffer stage medium. Pass through a suitable filter of 0.45-um pore size, discarding the first 4 mL of the filtrate. Dilute with Buffer stage medium to a concentration similar to that of the Buffer stage standard solution.
Acid stage blank: Use Acid stage medium.
Buffer stage blank: Use Buffer stage medium.
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy (857).)
Mode: UV
Analytical wavelength: 375 nm
Blank: Acid stage medium or Buffer stage medium
Analysis
Samples: Acid stage standard solution, Buffer stage standard solution, Acid stage sample solution, Buffer stage sample solution, Acid stage blank, and Buffer stage blank
Calculate the concentration (Ci) of nitrofurantoin (C8H6N4O5) in the sample withdrawn from the vessel at each time point (i):
Resulti = (AU/AS) × CS × D
AU = absorbance from the Acid stage sample solution or Buffer stage sample solution
AS = absorbance from the Acid stage stanard solution or Buffer stage standard solution
CS = 1 concentration of USP Nitrofurantoin RS in the Acid stage standard solution or Buffer stage standard solution (mg/mL)
D = dilution factor for the Acid stage sample solution or Buffer stage sample solution
Calculate the percentage of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved at each time point (i):
Result1 = C1 x V x (1/L) x 100
Result2 = [(C2 x V)+ (C1 x VSA)] x (1/L) x 100
Result3 = [(C3 x V) + [(C2 x VSB) x (C1 x VSA)] x (1/L) x 100
C = concentration of nitrofurantoin in the portion of sample withdrawn at time point / (mg/mL)
V = volume of the Acid stage medium or Buffer stage medium, 900 mL
L = label claim (mg/Capsule)
VSA = volume of the Acid stage sample solution withdrawn at time point 1 and replaced with Solution A, 50 mL
VSB = volume of the Buffer stage sample solution withdrawn at time point 2 and replaced with Buffer stage medium (mL)
Tolerances: See Table 15.
Table 15
| Time point (i) | Time (h) | Amount Dissolved (%) |
| 1 | 1 | 5–15 |
| 2 | 3 | 33–53 |
| 3 | 7 | NLT 80 |
The percentages of the labeled amount of nitrofurantoin (C8H6N4O5) dissolved at the specified times conform to Table 16.
Table 16
| Level | Number Tested | Criteria |
| L1 | 12 | The average value of the 12 units lies within each of the stated ranges and is NLT the stated amount at the final test time; none is >10% of labeled content outside each of the stated ranges; and none is >10% of the labeled content below the stated amount at the final test time. |
| L2 | 12 | The average value of the 24 units lies within each of the stated ranges and is NLT the stated amount at the final test time; NMT 2 of the 24 units are >10% of labeled content outside each of the stated ranges; NMT 2 of the 24 units are >10% of labeled content below the stated amount at the final test time; and none of the units are >20% of labeled content outside each of the stated ranges or >20% of the labeled content below the stated amount at the final test time. |
▲(RB 1-Aug-2023)
Change to read:
4.2 UNIFORMITY OF DOSAGE UNITS (905)
▲Meet the requirements ▲(Official 1-Aug-2023)
Procedure for content uniformity
Solution A, Mobile phase, Internal standard solution, Standard solution, Chromatographic system, and Analysis: Proceed as directed in the Assay.
Sample solution: Transfer the contents of 1 Capsule to a suitable flask, and add a volume of dimethylformamide to obtain a solution having a concentration of about 1.2 mg/mL of nitrofurantoin. Shake the flask for 15 min. If necessary, the sample may be homogenized, using a disperser. In the case of a 50- or 100-mg Capsule, transfer 40.0 mL of this solution to a suitable flask, add 50.0 mL of Internal standard solution, mix, and cool to room temperature. Pass a portion of the solution through a nylon filter of 0.45-µm pore size, discarding the first few mL of the filtrate. In the case of a 25-mg Capsule, transfer 20.0 mL of the solution to a suitable flask, and add 25.0 mL of Internal standard solution instead of 50.0 mL.
▲▲(Official 1-Aug-2023)
5 IMPURITIES
ORGANIC IMPURITIES: LIMIT OF NITROFURAZONE
Solution A: Prepare as directed in the Assay.
Mobile phase: Tetrahydrofuran and Solution A (1:9)
System suitability stock solution: 5.0 µg/mL each of nitrofurazone and nitrofurantoin in dimethylformamide
System suitability solution: System suitability stock solution and Mobile phase (1:10)
Standard stock solution: 5.0 µg/ml. of USP Nitrofurazone RS in dimethylformamide
Standard solution: Transfer 2.0 mL of the Standard stock solution into a glass-stoppered flask, add 20.0 mL of water, and mix.
Sample solution: Transfer a portion of Capsule contents equivalent to 100 mg of nitrofurantoin into a 25-mL glass-stoppered flask. Add 2.0 mL of dimethylformamide, and shake for 5 min. Add 20.0 mL of water, mix, and allow to stand for 15 min. Pass a portion of the mixture through a nylon filter of 0.45-um pore size.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 375 nm
Column: 3.9-mm x 30-cm; packing L1
Flow rate: 1.6 mL/min
Injection volume: 60-100 µL
System suitability
Samples: System suitability solution and Standard solution
[NOTE-Adjust the operating parameters so that the nitrofurazone peak in the chromatogram of the Standard solution has a retention time of about 10.5 min and a height of about 0.1 full-scale.]
Suitability requirements
Resolution: NLT 4.0 between the nitrofurazone and nitrofurantoin peaks, System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Acceptance criteria: The height of any peak from the Sample solution at a retention time corresponding to that of the main peak from the Standard solution is NMT the height of the main peak from the Standard solution. NMT 0.01% of nitrofurazone is found.
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers, and store at controlled room temperature.
LABELING: Capsules that contain the macrocrystalline form of nitrofurantoin are so labeled. When more than one Dissolution test is given, the labeling states the Dissolution test used only if Test 1 is not used.
USP REFERENCE STANDARDS (11)
USP Nitrofurantoin RS
USP Nitrofurazone RS


