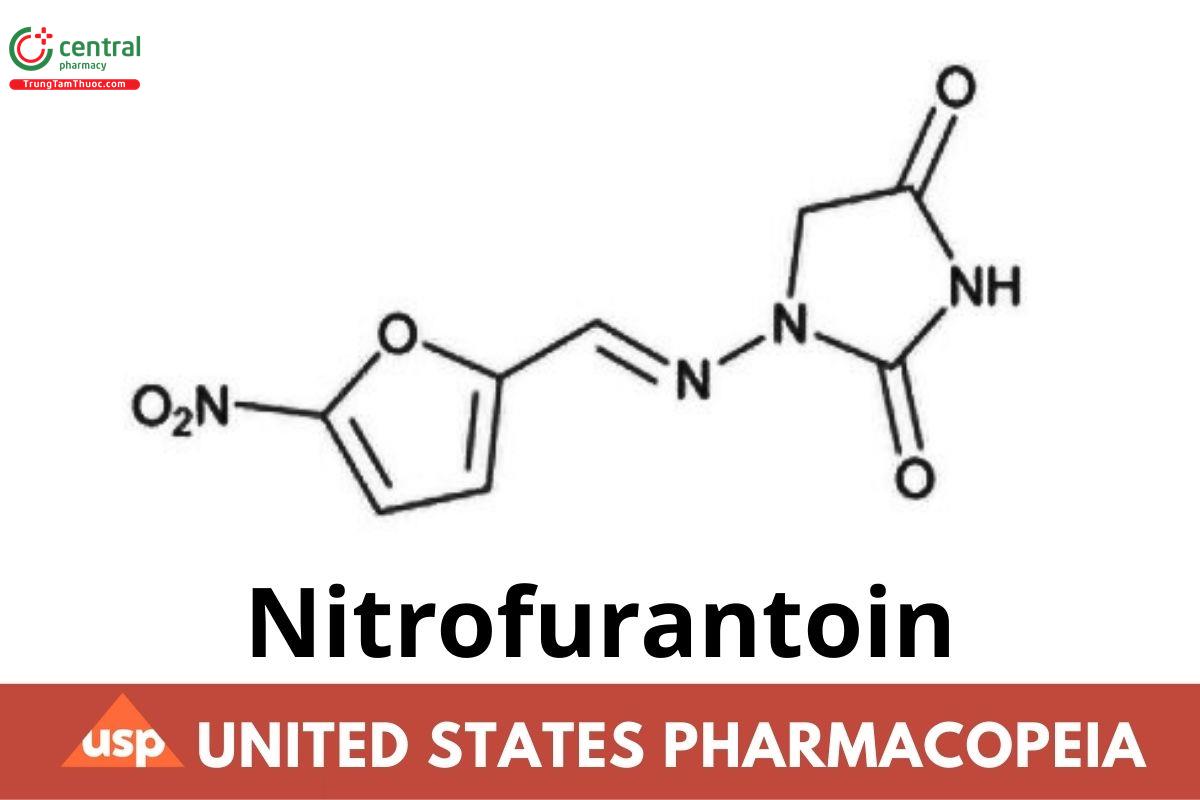
Nitrofurantoin
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C₈H₆N₄O₅ (anhydrous) 238.16
C₈H₆N₄O₅ · H₂O 256.18
2,4-Imidazolidinedione, 1-[[(5-nitro-2-furanyl)methylene] amino]-;
1-[(5-nitrofurfurylidene)amino]hydantoin CAS RN®: 67-20-9; UNII: 927AH8112L.
Monohydrate CAS RN®: 17140-81-7; UNII: E1QI2CQQ1I.
1 DEFINITION
Nitrofurantoin is anhydrous or contains one molecule of water of hydration. It contains NLT 98.0% and NMT 102.0% of C₈H₆N₄O₅, calculated on the anhydrous basis.
[Note-Nitrofurantoin and solutions of it are discolored by alkali and by exposure to light and are decomposed upon contact with metals other than stainless steel and aluminum.]
2 IDENTIFICATION
Change to read:
2.1 A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M
Sample: Previously dried at 140° for 30 min
Acceptance criteria: Meets the requirements
2.2 B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 Procedure
Buffer: Dissolve 6.8 g of monobasic potassium phosphate in 500 mL of water. Add about 30 mL of 1.0 N sodium hydroxide sufficient to adjust to a pH of 7.0, and dilute with water to 1 L.
Mobile phase: Acetonitrile and Buffer (12:88)
Internal standard solution: 1 mg/mL of acetanilide in water
Standard solution: 0.56 mg/mL of USP Nitrofurantoin RS prepared as follows. Dissolve 50 mg of USP Nitrofurantoin RS in 40.0 mL of dimethylformamide, and add 50.0 mL of Internal standard solution.
Sample solution: 0.56 mg/mL of Nitrofurantoin prepared as directed for Standard solution
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- [Note-Adjust the operating parameters so that the retention time of the nitrofurantoin peak is about 8 min and the peak heights are about half full-scale.]
- Mode: LC
- Detector: UV 254 nm
- Column: 3.9-mm × 30-cm; packing L1
- Injection size: 5–10 µL
System suitability
- Sample: Standard solution
- Suitability requirements
- Resolution: NLT 3.0 between the acetanilide and nitrofurantoin peaks
- Relative standard deviation: NMT 2.0% determined from the ratio of the peak responses
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of nitrofurantoin (C₈H₆N₄O₅) in the portion of Nitrofurantoin taken:
Result = (Rᵤ/Rₛ) × (Cₛ/Cᵤ) × 100
Rᵤ = internal standard ratio (peak response of nitrofurantoin/peak response of acetanilide) from the Sample solution
Rₛ = internal standard ratio (peak response of nitrofurantoin/peak response of acetanilide) from the Standard solution
Cₛ = concentration of USP Nitrofurantoin RS in the Standard solution (mg/mL)
Cᵤ = concentration of Nitrofurantoin in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the anhydrous basis
4 IMPURITIES
4.1 Limit of Nitrofurfural Diacetate
Standard solution: 100 µg/mL of USP Nitrofurfural Diacetate RS in dimethylformamide and acetone (1:10)
Sample solution: Dissolve 100 mg of Nitrofurantoin in 1 mL of dimethylformamide, and dilute with acetone to 10.0 mL.
Chromatographic system
- (See Chromatography 〈621〉, Thin-Layer Chromatography.)
- Mode: TLC
- Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
- Application volume: 10 µL
- Developing solvent system: Chloroform and methanol (9:1)
- Spray reagent: Dissolve 0.75 g of phenylhydrazine hydrochloride in 50 mL of water, and decolorize with activated charcoal. Add 25 mL of hydrochloric acid, and mix with water to produce 200 mL.
Analysis
Samples: Standard solution and Sample solution
Proceed as directed in the chapter. Develop in the Developing solvent system until the solvent front has moved three-fourths the length of the plate, allow to air-dry for 5 min, and heat the plate at 105° for 5 min. While it is still warm, locate the spots by spraying the plate with Spray reagent.
Acceptance criteria: Any spot produced by the Sample solution, at an R value of 0.7, is not greater in size or intensity than that produced by the Standard solution at the same R value: NMT 1.0% of nitrofurfural diacetate is found.
4.2 Limit of Nitrofurazone
Buffer: Prepare as directed in the Assay.
Mobile phase: Tetrahydrofuran and Buffer (10:90)
System suitability stock solution: 5.0 µg/mL each of USP Nitrofurazone RS and USP Nitrofurantoin RS in dimethylformamide
System suitability solution: 0.5 µg/mL each of USP Nitrofurazone RS and USP Nitrofurantoin RS in Mobile phase from System suitability stock solution
Standard stock solution: 5.0 µg/mL of USP Nitrofurazone RS in dimethylformamide
Standard solution: 0.5 µg/mL of USP Nitrofurazone RS in water from Standard stock solution
Sample solution: Dissolve 100 mg of Nitrofurantoin in 2.0 mL of dimethylformamide, and add 20.0 mL of water. Mix, and allow to stand for 15 min to allow a precipitate to form. Pass a portion of the solution through a suitable nylon filter of 0.45-µm pore size.
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- [Note-Adjust the operating parameters so that the retention time of the nitrofurazone peak is about 10.5 min and its height is about 0.1 half full-scale.]
- Mode: LC
- Detector: UV 375 nm
- Column: 3.9-mm × 30-cm; packing L1
- Flow rate: 1.6 mL/min
- Injection size: 60–100 µL
System suitability
- Samples: System suitability solution and Standard solution
- Suitability requirements
- Resolution: NLT 4.0 between the nitrofurazone and nitrofurantoin peaks, System suitability solution
- Relative standard deviation: NMT 2.0% determined from the peak height, Standard solution
Analysis
Samples: Standard solution and Sample solution
Acceptance criteria: The height of any peak appearing in the Sample solution at a retention time corresponding to that of the main peak from the Standard solution is NMT the height of the main peak from the Standard solution (NMT 0.01%).
5 SPECIFIC TESTS
5.1 Water Determination, Method III 〈921〉
Analysis: Dry a sample at 140° for 30 min.
Acceptance criteria: For the anhydrous form, it loses NMT 1.0% of its weight; for the hydrous form, it loses 6.5%–7.5% of its weight.
5.2 Specific Surface Area 〈846〉 (where it is labeled as being in the form of macrocrystals)
Sample: Outgas a portion of the powder to be placed under test at 150° for 10 min at ambient pressure with nitrogen.
Acceptance criteria: 0.045–0.20 m²/g
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers.
Labeling: Label it to indicate whether it is anhydrous or hydrous. Nitrofurantoin in the form of macrocrystals is so labeled. The labeling states the specific surface area and which method, specified under Specific Surface Area 〈846〉, is used.
USP Reference Standards 〈11〉
USP Nitrofurantoin RS
USP Nitrofurazone RS
USP Nitrofurfural Diacetate RS C9H9NO7 243.17


