Nifedipine
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
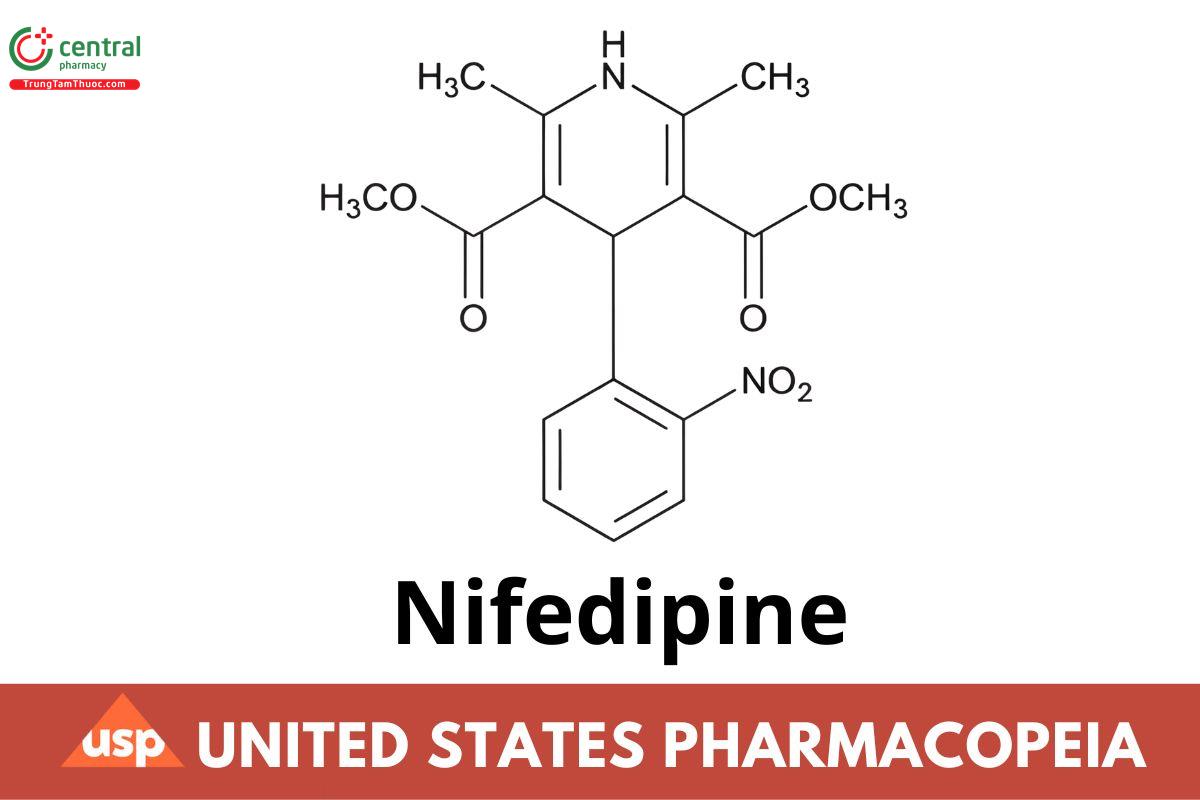
C17H18N2O6 346.33
3,5-Pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)-, dimethyl ester;
Dimethyl 1,4-dihydro-2,6-dimethyl-4-(o-nitrophenyl)-3,5-pyridinedicarboxylate CAS RN®: 21829-25-4; UNII: I9ZF7L6G2L.
1 DEFINITION
Nifedipine contains NLT 98.0% and NMT 102.0% of nifedipine (C17H18N2O6), calculated on the dried basis.
[NOTE-Nifedipine, when exposed to daylight and certain wavelengths of artificial light, readily converts to a nitrosophenylpyridine derivative. Exposure to UV light leads to the formation of a nitrophenylpyridine derivative. Perform the Assay and other tests in the dark or under golden fluorescent or other low-actinic light. Use low-actinic glassware.]
2 IDENTIFICATION
Change to read:
A. ▲SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K ▲(CN 1-MAY-2020): Do not dry samples.
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Protect the Standard solution and the Sample solution from actinic light. Conduct the Assay promptly after preparation of the Standard solution and the Sample solution.
Mobile phase: Acetonitrile, methanol, and water (25:25:50)
Standard stock solution: 1 mg/mL of USP Nifedipine RS in methanol
Standard solution: 0.1 mg/mL in Mobile phase from Standard stock solution
Sample solution: 0.1 mg/mL of Nifedipine prepared as follows. Transfer about 25 mg of Nifedipine to a 250-mL flask, dissolve in 25 mL of methanol, and dilute with Mobile phase to volume.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 235 nm
Column: 4.6-mm x 25-cm; 5-µm packing L1
Flow rate: 1 mL/min
Injection volume: 25 µL
System suitability
Sample: Standard solution
Suitability requirements
Column efficiency: NLT 4000 theoretical plates
Tailing factor: NMT 1.5
Relative standard deviation: NMT 1.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of nifedipine (C17H18N2O6) in the portion of Nifedipine taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Nifedipine RS in the Standard solution (mg/mL)
CU = concentration of Nifedipine in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-102.0% on the dried basis
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281)
NMT 0.1%, an ignition temperature of 600" being used
4.2 ORGANIC IMPURITIES
Protect Standard stock solution B and the Sample solution from actinic light. Conduct this test promptly after preparation of Standard stock solution B and the Sample solution.
Mobile phase, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Standard stock solution A: 1 mg/mL of USP Nifedipine RS in methanol
Standard solution A: 0.3 mg/mL of USP Nifedipine RS in Mobile phase from Standard stock solution A
Standard stock solution B: 1 mg/mL of USP Nifedipine Nitrophenylpyridine Analog RS in methanol
Standard solution B: 0.6 µg/mL of USP Nifedipine Nitrophenylpyridine Analog RS in Mobile phase from Standard stock solution B
Standard stock solution C: 1 mg/mL of USP Nifedipine Nitrosophenylpyridine Analog RS in methanol
Standard solution C: 0.6 µg/mL of USP Nifedipine Nitrosophenylpyridine Analog RS from Standard stock solution C, diluted with Mobile phase
Standard solution D: Mobile phase, Standard solution B, and Standard solution C (1:1:1)
System suitability solution: Standard solution A, Standard solution B, and Standard solution C (1:1:1)
System suitability
Sample: System suitability solution
Suitability requirements
Resolution: NLT 1.5 between nifedipine nitrophenylpyridine analog and nifedipine nitrosophenylpyridine analog; NLT 1.0 between the nifedipine nitrosophenylpyridine analog and nifedipine
Relative standard deviation: NMT 10% for both nifedipine nitrophenylpyridine analog and nifedipine nitrosophenylpyridine analog
Analysis
Samples: Sample solution and Standard solution D
Calculate the percentage of nifedipine nitrophenylpyridine analog and nifedipine nitrosophenylpyridine analog in the portion of Nifedipine taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of nifedipine nitrophenylpyridine analog or nifedipine nitrosophenylpyridine analog from the Sample solution
rS = peak response of nifedipine nitrophenylpyridine analog or nifedipine nitrosophenylpyridine analog from Standard solution D
CS = concentration of USP Nifedipine Nitrophenylpyridine Analog RS or USP Nifedipine Nitrosophenylpyridine Analog RS in Standard solution D (mg/mL)
CU = concentration of Nifedipine in the Sample solution (mg/mL)
Acceptance criteria: See Table 1.
Table 1
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Nifedipine nitrophenylpyridine analoga | 0.8 | 0.2 |
| Nifedipine nitrosophenylpyridine analogb | 0.9 | 0.2 |
| Nifedipine | 1.0 | — |
a Dimethyl 4-(2-nitrophenyl)-2,6-dimethylpyridine-3,5-dicarboxylate.
b Dimethyl 4-(2-nitrosophenyl)-2,6-dimethylpyridine-3,5-dicarboxylate.
4.3 LIMIT OF CHLORIDE AND SULFATE
Standard solution A: Add 5.0 mL of water to 10 mL of 8.2 µg/mL of sodium chloride (corresponding to 5 µg/mL of chloride).
Standard solution B: Equivalent to 10 µg/mL of sulfate from potassium sulfate in water
Sample solution: To 5.0 g of Nifedipine add 4.0 mL of 6 N acetic acid and 46 mL of water. Bring carefully to a boil on a hot plate. Cool, pass through filter paper that is free of chloride and sulfate, and use the filtrate.
Chloride: Pipet 2.5 mL of the Sample solution into a 50-mL color-comparison tube, and add 12.5 mL of water. Into a matched color-comparison tube, pipet 10 mL of Standard solution A. To each tube add 0.15 mL of 0.3 M nitric acid and 0.3 mL of silver nitrate TS, and mix.
Acceptance criteria: NMT 0.02%; the opalescence exhibited by the Sample solution does not exceed that of Standard solution A.
Sulfate: Pipet into each of two 50-mL matched color-comparison tubes 1.5 mL of Standard solution B. To each tube add, successively and with continuous shaking, 0.75 mL of alcohol, 0.5 mL of a 6.1% aqueous solution of barium chloride, and 0.25 mL of 6 N acetic acid. Shake for an additional 30 s. Pipet into one tube, designated the standard tube, 15 mL of Standard solution B. Pipet into the other tube, designated the sample tube, 3 mL of Sample solution and 12 mL of water.
Acceptance criteria: NMT 0.05%; the turbidity exhibited by the Sample solution in the sample tube does not exceed that of Standard solution B in the standard tube.
4.4 PERCHLORIC ACID TITRATION
Sample solution: Dissolve 4 g of Nifedipine in 160 mL of glacial acetic acid in a 250-mL conical flask with the aid of an ultrasonic bath.
Titrimetric system
Mode: Direct titration
Titrant: 0.1 N perchloric acid VS
Endpoint: Visual
Analysis: Add 3 drops of p-naphtholbenzein TS to the Sample solution, and titrate with Titrant to a green endpoint.
Acceptance criteria: NMT 0.12 mL of 0.1 N perchloric acid is consumed for each g of Nifedipine.
5 SPECIFIC TESTS
LOSS ON DRYING (731)
Analysis: Dry a sample at 105" to constant weight.
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight, light-resistant containers.
USP REFERENCE STANDARDS (11)
USP Nifedipine RS
USP Nifedipine Nitrophenylpyridine Analog RS
Dimethyl 4-(2-nitrophenyl)-2,6-dimethylpyridine-3,5-dicarboxylate.
C17H16N2O6 344.33
USP Nifedipine Nitrosophenylpyridine Analog RS
Dimethyl 4-(2-nitrosophenyl)-2,6-dimethylpyridine-3,5-dicarboxylate.
C17H16N2O5 328.33


