Nicotine Polacrilex
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
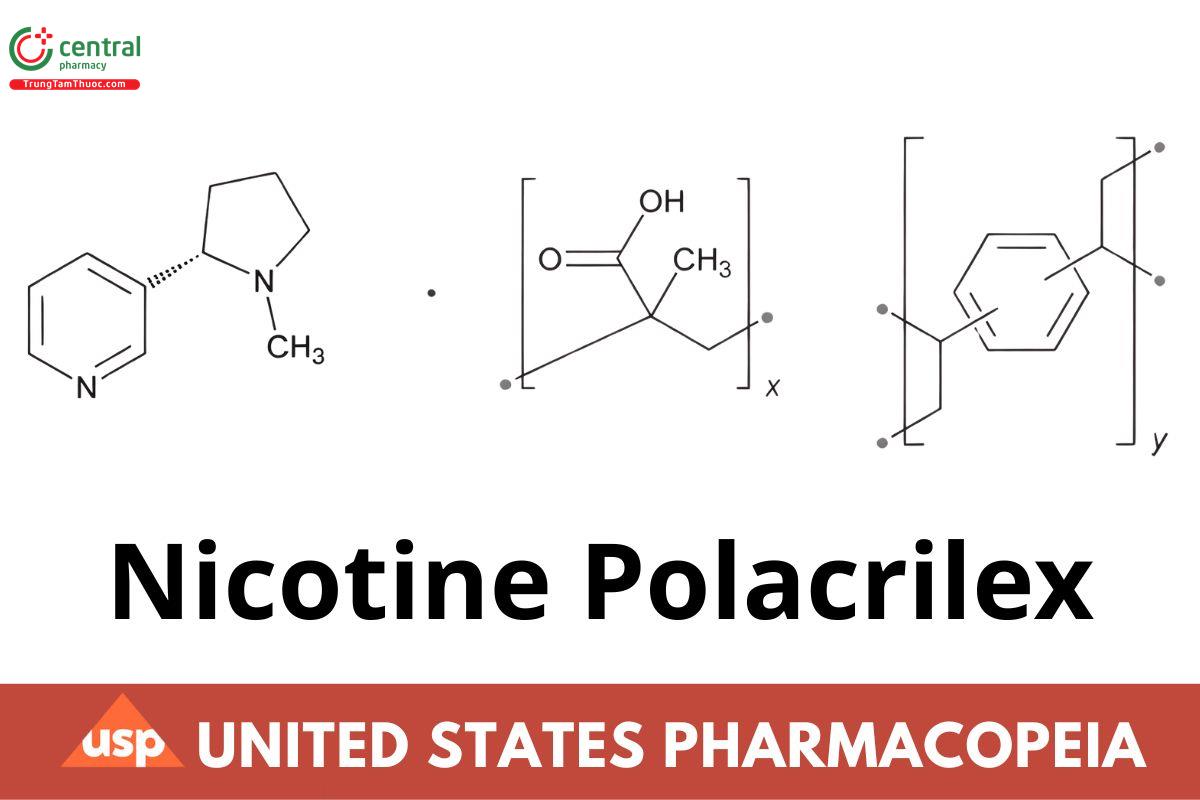
[(C4H6O2)x(C10H10)y] (C10H14N2)
2-Propenoic acid, 2-methyl-, polymer with diethenylbenzene, complex with (S)-3-(1-methyl-2-pyrrolidinyl)pyridine;
Methacrylic acid polymer with divinylbenzene, complex with nicotine CAS RN®: 96055-45-7.
1 DEFINITION
Nicotine Polacrilex is a weak carboxylic cation-exchange resin prepared from methacrylic acid and divinylbenzene, in complex with nicotine. It may contain glycerin. Glycerin-free Nicotine Polacrilex contains NLT 95.0% and NMT 115.0% of the labeled amount of nicotine (C10H14N2). calculated on the dried basis. Glycerin-containing Nicotine Polacrilex contains NLT 95.0% and NMT 115.0% of the labeled amount of nicotine (C10H14N2), calculated on the anhydrous basis.
[NOTE-Nicotine Polacrilex is also known as Nicotine Resinate.]
2 IDENTIFICATION
Change to read:
A. ▲SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy (for Nicotine): 197K or 197A ▲(CN 1-May-2020)
Sample: Transfer an amount of Nicotine Polacrilex equivalent to 100 mg of nicotine to a 100-mL glass-stoppered tube. Add 20 mL of 1 M ammonium hydroxide, 5 mL of 10 M sodium hydroxide, and 20 mL of n-hexane. Shake for 5 min, and allow the phases to separate. Transfer the upper hexane phase to an evaporating dish, and evaporate on a steam bath.
Standard: Use USP Nicotine Bitartrate Dihydrate RS, and prepare as directed for the Sample.
Acceptance criteria: Meets the requirements
Change to read:
B.▲SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy (for Polacrilex): 197K or 197A ▲(CN 1-May-2020)
Standard: Transfer a portion of USP Polacrilex Resin RS, equivalent to the amount of Nicotine Polacrilex used to prepare the Sample solution in the Assay, to a glass-stoppered tube. Add 10 mL of 1 M ammonium hydroxide, shake for 10 min, and then centrifuge. Decant the ammonia solution from the residue, and wash the residue by shaking it with three 10-mL volumes of water, decanting the water phase after each shaking. Wash with 10 mL of 0.1 N hydrochloric acid, decant the liquid, and dry the residue at 105°.
Sample: Use the residue obtained from the Sample solution in the Assay. Decant the ammonia solution remaining from the residue, and wash the residue by shaking it with three 10-mL volumes of water, decanting the water phase after each shaking. Wash with 10 mL of 0.1 N hydrochloric acid, decant the liquid, and dry the residue at 105°.
Acceptance criteria: Meets the requirements
C. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Solution A: Add 25 mL of 1 M acetic acid to 900 ml of water, and then add 6 mL of ammonium hydroxide. Adjust with either 2 M acetic acid or 2 M ammonium hydroxide to a pH of 10.0, and dilute with water to 1000 mL.
Solution B: Acetonitrile
Mobile phase: See Table 1. [NOTE-Re-equilibration time may be adjusted, if necessary.]
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 3 | 100 | 0 |
| 3.01 | 95 | 5 |
| 28 | 74 | 26 |
| 32 | 60 | 40 |
| 33 | 100 | 0 |
| 35 | 100 | 0 |
System suitability solution: 1.5 mg/mL of USP Nicotine Bitartrate Dihydrate RS and 6 µg/mL of USP Nicotine Related Compound G RS in water
Standard solution: 1.8 mg/mL of USP Nicotine Bitartrate Dihydrate RS in water
Sample solution: Nominally 0.6 mg/mL of nicotine prepared as follows. Transfer an amount of Nicotine Polacrilex equivalent to 30 mg of nicotine to a glass-stoppered tube. Add 10.0 mL of 1 M ammonium hydroxide, shake vigorously for 10 min, and then centrifuge. Transfer 5.0 mL of the clear solution to a 25-mL volumetric flask, add 5 mL of 1 M acetic acid, and dilute with water to volume. Retain the residue from centrifugation for use in Identification B.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm x 15-cm; 5-µm packing L1
Flow rate: 1.0 mL/min
Injection volume: 20 µL
System suitability
Samples: System suitability solution and Standard solution
[NOTE-See Table 2 for the relative retention times.]
Suitability requirements
Resolution: NLT 2.5 between nicotine and nicotine related compound G, System suitability solution
Tailing factor: NMT 2.0 for nicotine, System suitability solution
Relative standard deviation: NMT 1.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of nicotine (C10H14N2) in the portion of Nicotine Polacrilex taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
rU = peak response of nicotine from the Sample solution
rS = peak response of nicotine from the Standard solution
CS = concentration of USP Nicotine Bitartrate Dihydrate RS on the anhydrous basis in the Standard solution (mg/mL)
CU = nominal concentration of nicotine in the Sample solution (mg/mL)
Mr1 = molecular weight of nicotine, 162.23
Mr2 = molecular weight of anhydrous nicotine bitartrate, 462.41
Acceptance criteria:
Glycerin free: 95.0%-115.0% on the dried basis
Glycerin containing: 95.0%-115.0% on the anhydrous basis
4 PERFORMANCE TESTS
NICOTINE RELEASE
Solution A: 9 mg/mL of sodium chloride in water
Sample stock solution: Transfer an amount of Nicotine Polacrilex equivalent to 4 mg of nicotine to a glass-stoppered tube, add 10.0 mL of Solution A that has been warmed to 37°, and shake by mechanical means for 10 min. Immediately pass the liquid through a dry filter paper, discarding the first milliliter of the filtrate.
Sample solution: Transfer 1.0 mL of the Sample stock solution to a 25-mL volumetric flask, and dilute with 0.1. N hydrochloric acid to volume.
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy (857),)
Mode: UV
Analytical wavelengths: 236, 259, and 282 nm
Blank: 1.0 mL of Solution A diluted with 0.1 N hydrochloric acid to 25 mL
Analysis
Samples: Sample solution and Blank
Calculate the percentage of nicotine (C10H14N2) released:
Result = (A259 - 0.5A236 - 0.5A282) x (V/E) x (F/W) x (1/P) x 100
A259 = absorbance of the Sample solution, corrected for the Blank absorbance, at a wavelength of 259 nm
A236 = absorbance of the Sample solution, corrected for the Blank absorbance, at a wavelength of 236 nm
A282 = absorbance of the Sample solution, corrected for the Blank absorbance, at a wavelength of 282 nm
V = dilution volume, 250 mL
E = specific absorbance of nicotine at a wavelength of 259 nm, 323 mL g-1 cm-1
F = unit conversion factor, 1000 mg/g
W = weight of Nicotine Polacrilex (mg)
P = percentage of nicotine in Nicotine Polacrilex determined in the Assay
Acceptance criteria: NLT 70% in 10 min
5 IMPURITIES
ORGANIC IMPURITIES
Solution A, Solution B, Mobile phase, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
System suitability solution: 1.5 mg/mL of USP Nicotine Bitartrate Dihydrate RS and 6 µg/ml. each of USP Nicotine Related Compound A RS, USP Nicotine Related Compound B RS, USP Nicotine Related Compound C RS, USP Nicotine Related Compound D RS, USP Nicotine Related Compound E RS, USP Nicotine Related Compound FRS, and USP Nicotine Related Compound G. RS in water. [NOTE-The concentration of each related compound is in terms of the free base.]
Standard solution: 1.8 µg/mL of USP Nicotine Bitartrate Dihydrate RS in water
Sensitivity solution: 0.9 µg/mL of USP Nicotine Bitartrate Dihydrate RS in water from the Standard solution
System suitability
Samples: System suitability solution, Standard solution, and Sensitivity solution
[NOTE-See Table 2 for the relative retention times.]
Suitability requirements
Resolution: NLT 2.5 between nicotine and nicotine related compound G, System suitability solution
Tailing factor: NMT 2.0 for nicotine, System suitability solution
Relative standard deviation: NMT 5.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Sample solution and Standard solution
Calculate the percentage of each impurity in the portion of Nicotine Polacrilex taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of nicotine from the Standard solution
CS = concentration of USP Nicotine Bitartrate Dihydrate RS on the anhydrous basis in the Standard solution (mg/mL)
CU = nominal concentration of nicotine in the Sample solution (mg/mL)
Mr1 = molecular weight of nicotine, 162.23
Mr2 = 1 molecular weight of anhydrous nicotine bitartrate, 462.41
Acceptance criteria: See Table 2. The reporting threshold is 0.05%.
Table 2
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Nicotine related compound E | 0.3 | 0.3 |
| Nicotine related compound C | 0.55 | 0.3 |
| Nicotine related compound F | 0.7 | 0.3 |
| Nicotine related compound A | 0.8 | 0.3 |
| Nicotine related compound D | 0.86 | 0.3 |
| Nicotine related compound G | 0.9 | 0.3 |
| Nicotine | 1.00 | — |
| Nicotine related compound B | 1.6 | 0.3 |
| Any other unspecified impurity | — | 0.10 |
| Total impurities | — | 0.8 |
6 SPECIFIC TESTS
WATER DETERMINATION (921), Method / (for glycerin-containing Nicotine Polacrilex)
Sample solution: Transfer about 1.0 g of Nicotine Polacrilex to a 50-mL glass-stoppered test tube, and add 20.0 mL of methanol. Shake for 30 min, and allow to stand for 30 min. Use a 10-mL portion of the methanol layer for the titration.
Acceptance criteria: NMT 5.0%
LOSS ON DRYING (731), (for glycerin-free Nicotine Polacrilex)
Analysis: Dry at 105° for 2 h.
Acceptance criteria: NMT 7.0%
7 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers, protected from light.
LABELING: Label to indicate if the article contains glycerin.
USP REFERENCE STANDARDS (11)
USP Nicotine Bitartrate Dihydrate RS
USP Nicotine Related Compound A RS
Anatabine;
1,2,3,6-Tetrahydro-2,3'-bipyridine.
C10H12N2 160.22
USP Nicotine Related Compound B. RS
3-(1-Methyl-1H-pyrrol-2-yl)pyridine; Also known as Nicotyrine.
C10H10N2 158.20
USP Nicotine Related Compound. C. RS
(S)-1-Methyl-5-(pyridin-3-yl)pyrrolidin-2-one; Also known as Cotinine.
C10H12N2O 176.22
USP Nicotine Related Compound D. RS
3-(4,5-Dihydro-3H-pyrrol-2-yl)pyridine fumarate; Also known as Myosamine.
C9H10N2O · C2H2O4 262.26
USP Nicotine Related Compound ERS
(1RS,2S)-1-Methyl-2-(pyridin-3-yl)pyrrolidine 1-oxide oxalate; Also known as Nicotine N-oxide.
C10H14N2O · C2H2O4 268.27
USP Nicotine Related Compound FRS
3-(Pyrrolidin-2-yl)pyridine; Also known as Nornicotine.
C9H12N2 148.20
USP Nicotine Related Compound G.RS
(S)-3-(Piperidin-2-yl)pyridine; Also known as Anabasine.
C10H14N2 162.23
USP Polacrilex Resin RS


