Neotame
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
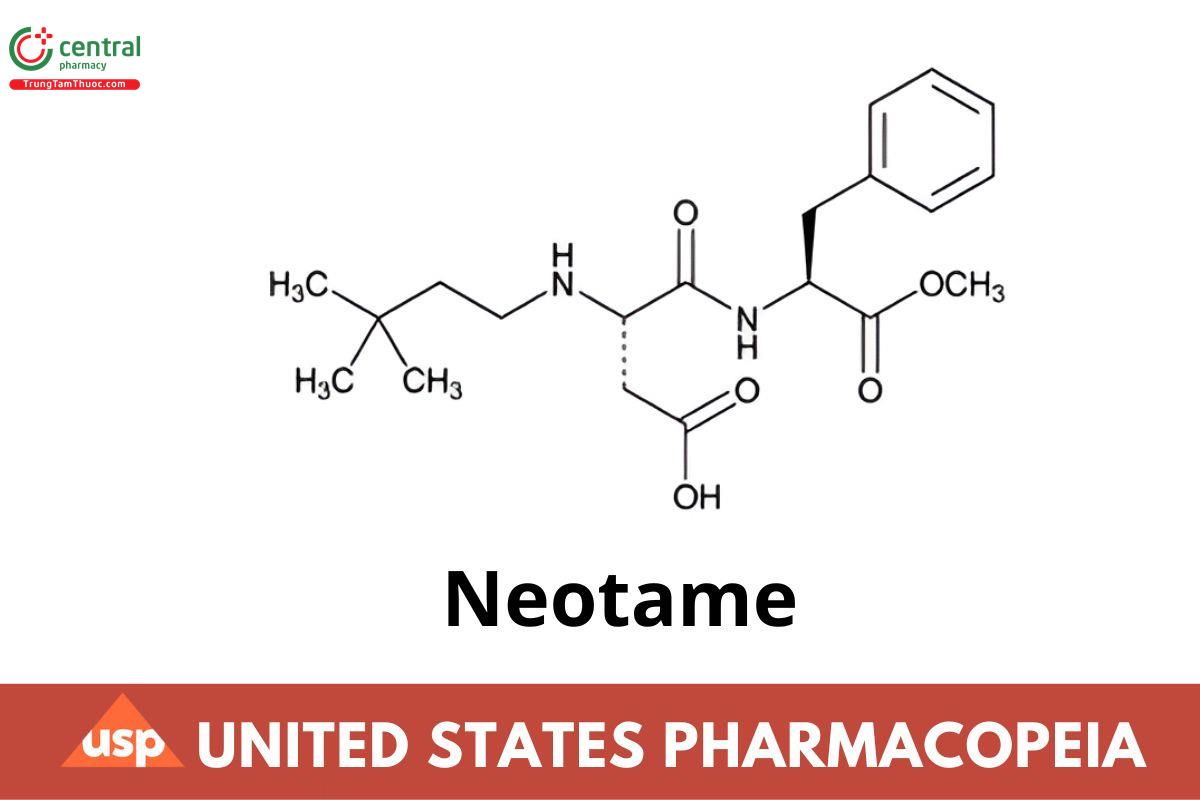
C20H30N2O5 378.46
l-Phenylalanine, N-[N-(3,3-dimethylbutyl)-l-α-aspartyl]-1-methyl ester;
N-[N-(3,3-Dimethylbutyl)-l-α-aspartyl]-l-phenylalanine 1-methyl ester CAS RN®: 165450-17-9.
1 DEFINITION
Neotame contains NLT 97.0% and NMT 102.0% of neotame (C20H30N2O5), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
3 ASSAY
Procedure
Mobile phase: Dissolve 3.0 g of sodium 1-heptanesulfonate in 740 mL of water in a suitable 1000-mL vessel, and add 3.8 mL of triethylamine. Adjust the resulting solution with phosphoric acid to a pH of 3.5, and dilute with water to 750 mL. Add 250 mL of acetonitrile, and adjust with phosphoric acid to an apparent pH of 3.7.
Standard solution: 1.0 mg/mL of USP Neotame RS in Mobile phase
Sample solution: 1.0 mg/mL of Neotame in Mobile phase. [Note—This solution is stable for up to 32 h when stored at a temperature of 0°–10°.]
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 210 nm
Column: 4.6-mm × 10-cm; packing L1
Column temperature: 45°
Flow rate: 1.5 mL/min
Injection volume: 25 μL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of neotame (C20H30N2O5) in the portion of Neotame taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Neotame RS in the Standard solution (mg/mL)
CU = concentration of Neotame in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–102.0% on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.2%
Lead
[Note - Use acid-cleaned (mixture of 5% nitric acid and 5% hydrochloric acid followed by rinsing with water) autosampler cups and volumetric glassware to avoid contamination. For the preparation of all aqueous solutions and for the rinsing of glassware before use, use water that has been passed through a strong-acid, strong-base, mixed-bed ion-exchange resin. Select all reagents to have as low a content of lead as practicable. Store standards and samples in acid-cleaned polyethylene containers.]
Diluent: Transfer 2 mL of lead-free nitric acid into a 1000-mL volumetric flask, dilute with water to volume, and mix.
Standard stock solution: 79.9 mg of lead nitrate in 100 mL of Diluent in a 500-mL volumetric flask, and dilute with Diluent to volume. Transfer 10.0 mL of the resulting solution into a 100-mL volumetric flask, and dilute with Diluent to volume. Each mL of the Standard stock solution contains the equivalent of 10 μg of lead.
Standard solution A: Dilute an aliquot of the Standard stock solution with Diluent to obtain a solution having a concentration of 0.03 μg/mL.
Standard solution B: Dilute an aliquot of the Standard stock solution with Diluent to obtain a solution having a concentration of 0.015 μg/mL.
Sample solution: Transfer 160 mg of Neotame to a 10-mL volumetric flask. Dissolve in and dilute with Diluent to volume.
Blank: Diluent
Instrumental conditions
(See Atomic Absorption Spectroscopy 〈852〉.)
[Note - Optimize the instrument program as recommended by the manufacturer for lead, using a char temperature of 500° and an atomization temperature of 2000°.]
Mode: Atomic absorption spectrophotometer with a graphite furnace, pyrolytically coated graphite tubes, a solid pyrolytic graphite platform, and a background compensation system
Analytical wavelength: 283.3 nm
Lamp: Lead hollow-cathode
Purge gas: Argon
Alternate gas: Breathing-quality air
Volume: 15 μL
Analysis
Samples: Standard solution A, Standard solution B, Sample solution, and Blank
Correct the peak areas of the Sample solution, Standard solution A, and Standard solution B for the Blank peak area. Generate the appropriate lead calibration algorithm, and determine the lead concentration in the Sample solution, in μg/mL.
Calculate the amount of lead, in μg/g, in the portion of Neotame taken:
Result = (C × V)/(W × F)
C = blank-corrected lead concentration in the Sample solution (μg/mL)
V = volume of the Sample solution, 10 mL
W = weight of Neotame taken to prepare the Sample solution (mg)
F = conversion factor, mg/g
Acceptance criteria: NMT 2 μg/g
Related Compounds
Mobile phase and Chromatographic system: Proceed as directed in the Assay.
Standard solution A: 0.03 mg/mL of USP Neotame Related Compound A RS in Mobile phase
Standard solution B: Prepare as directed for the Standard solution in the Assay.
Detector sensitivity solution: Transfer 2 mL of Standard solution A to a 50-mL volumetric flask, and dilute with Mobile phase to volume.
Sample solution: 2 mg/mL of Neotame in Mobile phase. [Note—This solution is stable for up to 32 h when stored at a temperature of 0°–10°.]
System suitability
Samples: Standard solution A and Detector sensitivity solution
Suitability requirements
Relative standard deviation: NMT 5.0%, Standard solution A
Signal-to-noise ratio: NLT 10, Detector sensitivity solution
Analysis
Samples: Standard solution A, Standard solution B, and Sample solution
Calculate the percentage of neotame related compound A in the portion of Neotame taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of neotame related compound A from the Sample solution
rS = peak response of neotame related compound A from Standard solution A
CS = concentration of USP Neotame Related Compound A RS in Standard solution A (mg/mL)
CU = concentration of Neotame in the Sample solution (mg/mL)
Calculate the percentage of other impurities in the portion of Neotame taken:
Result = (rT/rS) × (CS/CU) × 100
rT = sum of the peak responses of all impurities (except those of neotame related compound A and the solvent, if observed) from the Sample solution
rS = peak response of neotame from Standard solution B
CS = concentration of USP Neotame RS in Standard solution B (mg/mL)
CU = concentration of Neotame in the Sample solution (mg/mL)
Acceptance criteria
Neotame related compound A: NMT 1.5%
Other impurities: NMT 2.0%
5 SPECIFIC TESTS
Optical Rotation 〈781S〉, Procedures, Specific Rotation
Sample solution: 5 mg/mL in water
Acceptance criteria: −40.0° to −43.4°, at 20°
Water Determination 〈921〉, Method I, Method Ia
Sample: 0.50 g
Acceptance criteria: NMT 5.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers, store in a dry place, and avoid exposure to excessive heat.
USP Reference Standards 〈11〉
USP Neotame RS
USP Neotame Related Compound A RS
N-[N-(3,3-Dimethylbutyl)-l-α-aspartyl]-l-phenylalanine.


