Neomycin Sulfate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
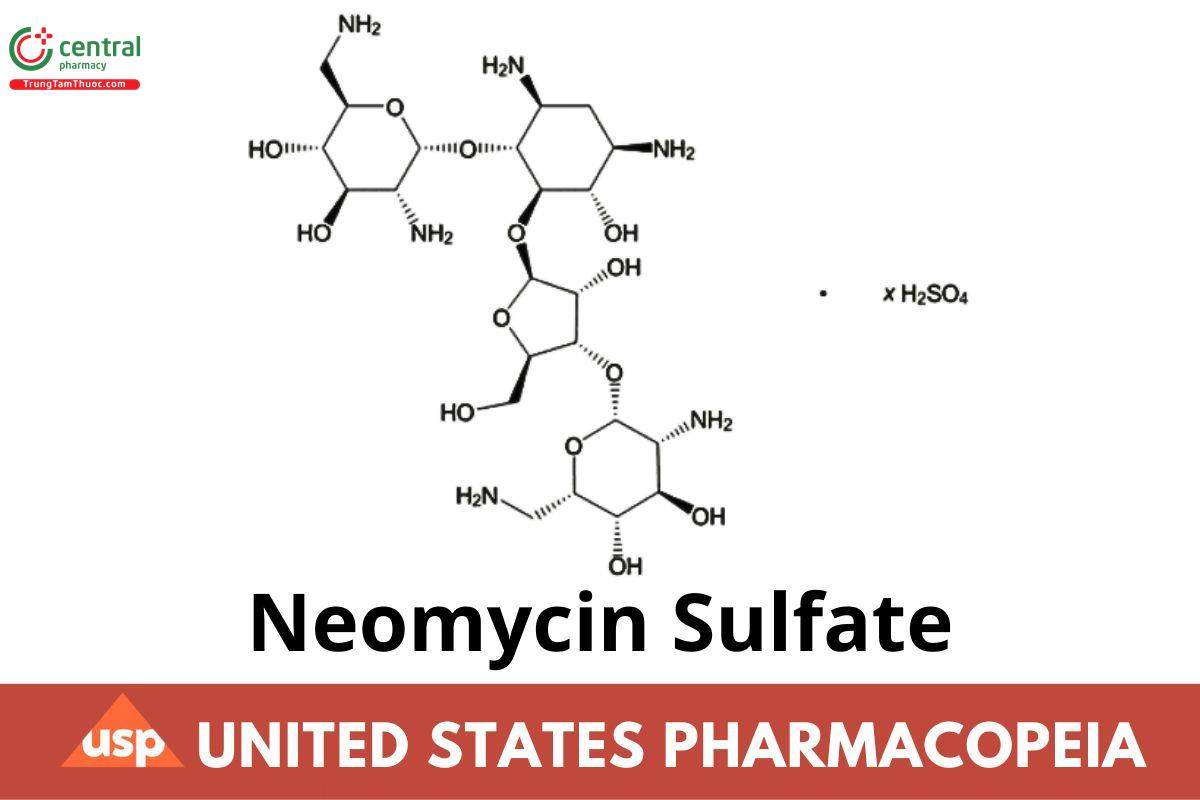
C23H46N6O13 (free base) 614.65 (free base)
Neomycin B
2-Deoxy-4-O-(2,6-diamino-2,6-dideoxy-α-d-glucopyranosyl)-5-O-[3-O-(2,6-diamino-2,6-dideoxy-β-l-idopyranosyl)-β-d-ribofuranosyl]-d-streptamine (free base) CAS RN: 119-04-0. (USP 1-Aug-2023)
Neomycin sulfate CAS RN: 1405-10-3; UNII: 057Y626693.
Change to read:
1 DEFINITION
Neomycin Sulfate is the sulfate salt of a kind of neomycin, an antibacterial substance produced by the growth of Streptomyces fradiae Waksman (Fam. Streptomycetaceae), or a mixture of two or more such salts, the main component being neomycin B sulfate. (USP 1-Aug-2023) It has a potency equivalent to NLT 600 µg of neomycin/mg, calculated on the dried basis.
2 IDENTIFICATION
A. It meets the requirements for neomycin under Thin-Layer Chromatographic Identification Test (201BNP).
Change to read:
B.
Mobile phase, System suitability solution, Sensitivity solution, Standard solution, Chromatographic system, and System suitability: Proceed as directed in the test for Composition of Neomycin Sulfate.
Sample identification solution: 0.025 mg/mL of Neomycin Sulfate in Mobile phase
Acceptance criteria: The retention time of the neomycin B peak of the Sample identification solution corresponds to that of the Standard solution, as obtained in the test for Composition of Neomycin Sulfate (USP 1-Aug-2023)
C. IDENTIFICATION TESTS-GENERAL (191), Chemical Identification Tests, Sulfate
Sample solution: 50 mg/mL
Acceptance criteria: Meets the requirements
3 ASSAY
PROCEDURE
(See Antibiotics-Microbial Assays (81).)
Analysis: Proceed as directed in the chapter.
Acceptance criteria: NLT 600 µg of neomycin/mg on the dried basis
Add the following:
4 IMPURITIES
ORGANIC IMPURITIES
Mobile phase, System suitability solution, Sensitivity solution, Standard solution, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the test for Composition of Neomycin Sulfate.
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each individual impurity in the portion of Neomycin Sulfate taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response of each individual impurity from the Sample solution
rs = peak response of neomycin B from the Standard solution
Cs = concentration of USP Neomycin B RS in the Standard solution (mg/mL)
Cu = concentration of neomycin sulfate in the Sample solution (mg/mL)
Acceptance criteria: See Table 1. The reporting threshold is 1.0%.
Table 1
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Neomycin B-LPa | 1.1 | - |
| Any individual unspecied impurity | - | 5.0 |
| Total unspecied impurities | - | 15.0 |
a 3-N-Acetyl-2-deoxy-4-O-(2,6-diamino-2,6-dideoxy-α-d-glucopyranosyl)-5-O-[3-O-(2,6-diamino-2,6-dideoxy-β-l-idopyranosyl)-β-dribofuranosyl]-d-streptamine.
(USP 1-Aug-2023)
5 SPECIFIC TESTS
Add the following:
COMPOSITION OF NEOMYCIN SULFATE
Mobile phase: 20 mL of trifluoroacetic acid, 6 ml of 50% sodium hydroxide TS, and 500 mL of water, diluted with water to 1000 ml.
System suitability solution: 0.1 mg/mL of USP Neomycin Sulfate System Suitability Mixture RS (contains neomycin B and neomycin C) in Mobile phase
Sensitivity solution: 0.005 mg/mL of USP Neomycin B RS in Mobile phase
Standard solution: 0.025 mg/mL of USP Neomycin B. RS and 0.01 mg/mL of USP Neomycin A RS in Mobile phase
Sample solution: 0.5 mg/mL of Neomycin Sulfate in Mobile phase
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: Pulsed amperometric electrochemical
Electrodes
Indicator: Gold
Reference: Silver-silver chloride
Auxillary: Stainless steel (cell body)
Waveform: See Table 2.
Table 2
| Time (s) | Potential (V) | Integration |
| 0.00 | +0.00 | - |
| 0.10 | +0.00 | Begin |
| 0.40 | +0.00 | End |
| 0.41 | +0.80 | - |
| 0.55 | +0.80 | - |
| 0.56 | −0.60 | - |
| 1.00 | −0.60 | - |
Column: 4.6-mm × 25-cm; 5-µm packing L1
Flow rate: 0.7 mL/min
Post-column reagent: 20 g/L of sodium hydroxide (carbonate-free), prepared from 50% sodium hydroxide TS in water, carbon dioxide-free.
This solution is added pulselessly to the column euent using a 375-µL polymeric mixing coil.1
Flow rate of post-column reagent: 0.5 mL/min
[Note—Adjust accordingly to meet the System suitability requirements.]
Injection volume: 10 µL
Run time: NLT 1.5 times the retention time of neomycin B
System suitability
Samples: System suitability solution, Sensitivity solution, and Standard solution
Suitability requirements
Resolution: NLT 2.0 between the neomycin C and neomycin B peaks, System suitability solution
Relative standard deviation: NMT 5.0% each for neomycin A and neomycin B, Standard solution
Signal-to-noise ratio: NLT 10 for the neomycin B peak, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of neomycin A in the portion of Neomycin Sulfate taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response of neomycin A from the Sample solution
rs = peak response of neomycin A from the Standard solution
Cs = concentration of USP Neomycin A RS in the Standard solution (mg/mL)
Cu = concentration of neomycin sulfate in the Sample solution (mg/mL)
Calculate the percentage of neomycin C in the portion of Neomycin Sulfate taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response of neomycin C from the Sample solution
rs = peak response of neomycin B from the Standard solution
Cs = concentration of USP Neomycin B RS in the Standard solution (mg/mL)
Cu = concentration of neomycin sulfate in the Sample solution (mg/mL)
Acceptance criteria: See Table 3.
Table 3
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Neomycin A | 0.7 | 2.0 |
| Neomycin Ca | 0.9 | 3.0–15.0 |
| Neomycin B | 1.0 | - |
a 2-Deoxy-4-O-(2,6-diamino-2,6-dideoxy-α-d-glucopyranosyl)-5-O-[3-O-(2,6-diamino-2,6-dideoxy-α-d-glucopyranosyl)-β-d-ribofuranosyl]-dstreptamine.
(USP 1-Aug-2023)
LOSS ON DRYING (731)
Sample: About 100 mg
Analysis: Dry the Sample under vacuum at a pressure not exceeding 5 mm of mercury at 60° for 3 h.
Acceptance criteria: NMT 8.0%
PH (791)
Sample solution: 33 mg/mL of neomycin
Acceptance criteria: 5.0-7.5
Change to read:
STERILITY TESTS (71): (USP 1-Aug-2023) Where the label states that Neomycin Sulfate is sterile, it meets the requirements under the relevant (USP 1-Aug-2023) dosage form.
(USP 1-Aug-2023)
Change to read:
BACTERIAL ENDOTOXINS TEST (85) (USP 1-AUG-2023) Where the label states that Neomycin Sulfate is sterile or that it must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement under the relevant dosage form monograph(s) in which neomycin is used can be met.
(USP 1-Aug-2023)
6 ADDITIONAL REQUIREMENTS
Change to read:
PACKAGING AND STORAGE: Preserve in tight, light-resistant containers. Store at controlled room temperature (USP 1-Aug-2023)
LABELING: Where it is intended for use in preparing injectable or other sterile dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable or other sterile dosage forms.
Change to read:
USP REFERENCE STANDARDS (11)
USP Neomycin Sulfate RS
USP Neomycin AF RS
2-Deoxy-4-O-(2,6-diamino-2,6-dideoxy-α-d-glucopyranosyl)-d-streptamine (free base).
C12H26N4O6 (free base) 322.36 (free base)
USP Neomycin Sulfate System Suitability Mixture RS
A mixture of neomycin 8 sulfate and neomycin C sulfate. (USP 1-Aug-2023)
1 A suitable mixing coil is the knitted reaction coil, part #043700, available from Dionex Corporation.


