Naratriptan Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
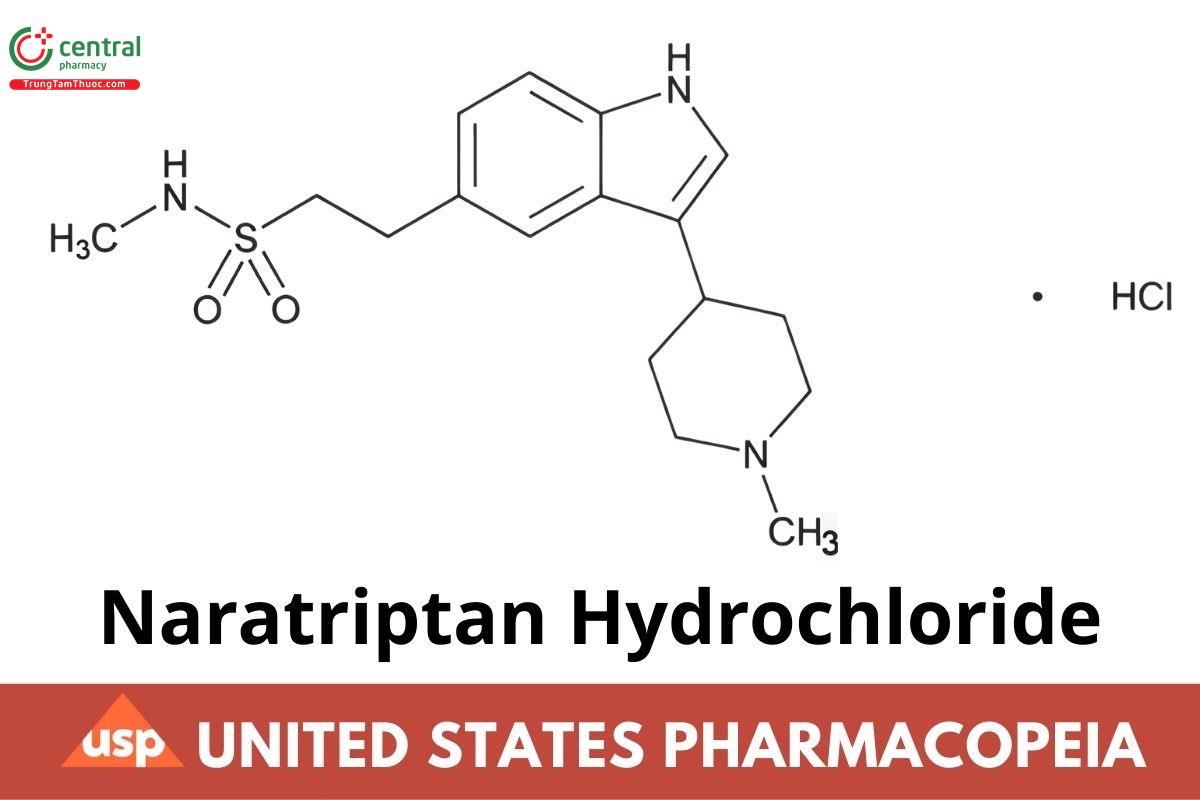
C17H25N3O2S · HCl 371.93
1H-Indole-5-ethanesulfonamide, N-methyl-3-(1-methyl-4-piperidinyl)-, monohydrochloride;
N-Methyl-3-(1-methyl-4-piperidyl)indole-5-ethanesulfonamide monohydrochloride CAS RN®: 143388-64-1; UNII: 10X8X4P12Z.
Change to read:
1 DEFINITION
Naratriptan Hydrochloride contains NLT 98.0% and NMT 101.0% of naratriptan hydrochloride (C17H25N3O2S · HCl), calculated on the anhydrous and solvent-free basis.
▲▲(USP 1-May-2021)
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197M
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
Delete the following:
▲ C. IDENTIFICATION TESTS-GENERAL (191), Chloride: It meets the requirements of test C. ▲(USP 1-May-2021)
Add the following:
▲C. CHLORIDE
CAUTION-This test evolves chlorine gas.]
Sample: Mix the solid under test with an equal weight of manganese dioxide and moisten with sulfuric acid.
Analysis: Gently heat the Sample, while exposing moistened starch iodide paper to the evolving gas.
Acceptance criteria: A gas evolves producing a blue color on the paper. ▲(USP 1-May-2021)
3 ASSAY
Change to read:
PROCEDURE
▲[NOTE-Store the System suitability solution, Standard solution, and Sample solution in a cool place, protected from light. ▲(USP 1-May-2021)
Solution A: Dilute 0.6 mL of phosphoric acid with water to 900 mL. Adjust with triethylamine to a pH of 2.5.
Mobile phase: Isopropyl alcohol and Solution A (1:9)
System suitability solution: 0.7 mg/mL of USP Naratriptan Resolution Mixture RS in Mobile phase
Standard solution: 0.11 mg/ml. of USP Naratriptan Hydrochloride RS in Mobile phase
Sample solution: 0.11 mg/mL of Naratriptan Hydrochloride in Mobile phase
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 282 nm
Column: 4.6-mm x 15-cm; 3-µm packing L11
Column temperature: 35°
Flow rate: 1.5 mL/min.
Injection volume: 10 µL
▲Run time: NLT 2 times the retention time of naratriptan ▲(USP 1-May-2021)
System suitability
Samples: System suitability solution and Standard solution
[NOTE-The relative retention times for naratriptan related compound A, naratriptan, and naratriptan related compound B are 0.9, 1.0, and 1.1, respectively.]
Suitability requirements
Resolution: NLT 1.5 between the naratriptan related compound A and naratriptan peaks; NLT 1.5 between the naratriptan and naratriptan related compound B peaks, System suitability solution
Relative standard deviation: ▲NMT 0.73%, ▲(USP 1-May-2021) Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of naratriptan hydrochloride (C17H25N3O2S · HCl) in the portion of Naratriptan Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Naratriptan Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Naratriptan Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-101.0% on the anhydrous and solvent-free basis
4 IMPURITIES
Change to read:
ORGANIC IMPURITIES
▲[NOTE-Store the System suitability solution and Sample solution in a cool place, protected from light.) ▲(USP 1-May-2021)
Buffer: 5.75 g/L of monobasic ammonium phosphate in water. Adjust with phosphoric acid to a pH of 3.00 ± 0.05.
Solution A: Acetonitrile and Buffer (3:97)
Solution B: Acetonitrile and Buffer (20:80)
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 35 | 0 | 100 |
| 40 | 0 | 100 |
| 41 | 100 | 0 |
| 51 | 100 | 0 |
System suitability solution: 0.11 mg/mL of USP Naratriptan Resolution Mixture RS in water
Sample solution: 0.11 mg/mL of Naratriptan Hydrochloride in water
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 225 nm
Column: 4.6-mm x 15-cm; 4-µm packing L1
Column temperature: 40°
Flow rate: 1.5 mL/min
Injection volume: 20 µL
System suitability
Sample: System suitability solution
[NOTE-See Table 2 for relative retention times.]
Suitability requirements
Resolution: NLT 1.5 between naratriptan and naratriptan related compound B
Analysis
Sample: Sample solution
Calculate the percentage of any individual impurity in the portion of Naratriptan Hydrochloride taken:
Result = {(rU/F)/[rN + Σ(rU/F)]} × 100
rU = peak response of each impurity from the Sample solution
F = relative response factor (see Table 2)
rN = peak response of naratriptan from the Sample solution
Acceptance criteria: See Table 2.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| ▲Naratriptan related compound A ▲(USP 1-May-2021) | 0.93 | 1.0 | 0.2 |
| Naratriptan | 1.00 | — | — |
| ▲Naratriptan related compound BA ▲(USP 1-May-2021) | 1.04 | 0.6 | 0.1 |
| ▲Bisaryl naratriptana ▲(USP 1-May-2021) | 1.18 | 0.6 | 0.2 |
| ▲Naratriptan pyridiniumb ▲(USP 1-May-2021) | 1.25 | 0.4 | 0.2 |
| ▲N-Sulfamoylethyl naratriptanc ▲(USP 1-May-2021) | 1.36 | 0.6 | 0.3 |
| ▲N-Sulfamoylethyl naratriptan pyridiniumd ▲(USP 1-May-2021) | 1.44 | 0.5 | 0.1 |
| ▲N-Sulfamoylethyl naratriptan amidee ▲(USP 1-May-2021) | 1.48 | 1.0 | 0.2 |
| ▲Naratriptan ethyl analogf▲(USP 1-May-2021) | 1.90 | 1.0 | 0.2 |
| Any other individual impurity | — | 1.0 | 0.1 |
| Total impurities | — | — | 1.5 |
a N-Methyl-2,2-bis[3-(1-methylpiperidin-4yl)-1H-indol-5-yl)ethanesulfonamide; Also known as 2,2-Bis-[3-(1-methylpiperidin-4-yl)-1H-indol-5-yl]ethanesulfonic acid methylamide.
b 1-Methyl-4-(5-[2-(N-methylsulfamoyl)ethyl]-1H-indol-3-yl)pyridin-1-ium; Also known as 1-Methyl-4-[5-(2-methylsulfamoyl-ethyl)-1H-indol-3-yl]-pyridinium.
c 2,2'-[3-(1-Methylpiperidin-4-yl)-1H-indole-1,5-diyl)bis(N-methylethanesulfonamide); Also known as 2-(3-(1-Methylpiperidin-4-yl)-5-(2-methylsulfamoyl-ethyl)-indol-1-yl]ethanesulfonic acid methylamide.
d 4-[1,5-Bis(2-(N-methylsulfamoyl)ethyl)-1H-indol-3-yl]-1-methylpyridin-1-ium; Also known as 4-[1,5-Bis-(2-methylsulfamoyl-ethyl)-1H-indol-3-yl]-1-methylpyridinium.
e N-Methyl-2-13-(1-methylpiperidin-4-yl)-1H-indol-5-yl]-N-[2-(N-methylsulfamoyl)ethyl]ethanesulfonamide; Also known as 2-[3-(1-Methylpiperidin-4-yl)-1H-indol-5-yl]ethane-sulfonic acid methyl-(2-methylsulfamoyl-ethyl)amide.
f 5-Ethyl-3-(1-methylpiperidin-4-yl)-1H-indole.
5 SPECIFIC TESTS
WATER DETERMINATION (921), Method : NMT 0.5%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers, and store below 30".
USP REFERENCE STANDARDS (11)
USP Naratriptan Hydrochloride RS
USP Naratriptan Resolution Mixture RS
Contains a mixture of Naratriptan Hydrochloride with approximately 0.1% each of the following compounds:
Naratriptan related compound A: [3-(1-Methylpiperidin-4-yl)-1H-indole hydrochloride].
Naratriptan related compound B: [2-[3-(1-Methyl-1,2,3,6-tetrahydropyridin-4-yl)-1H-indol-5-yl]ethanesulfonic acid methylamide oxalate].


