Naltrexone Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
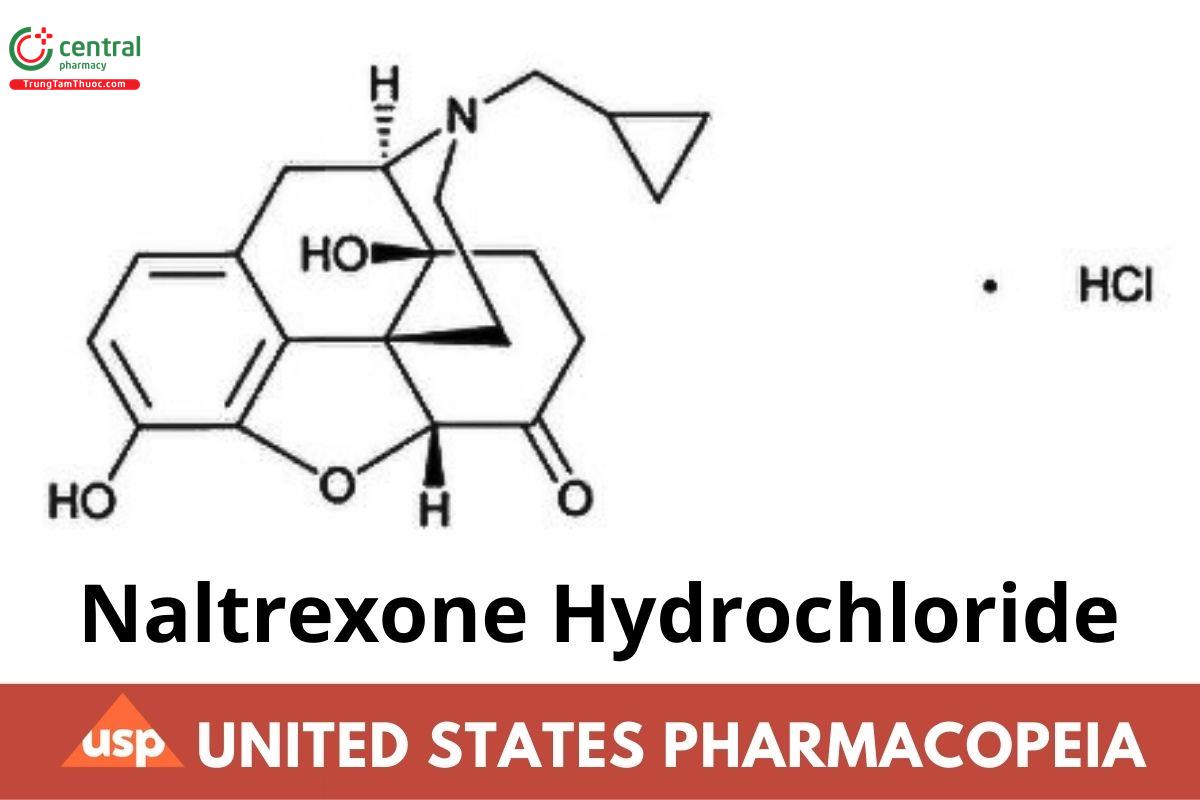
C₂₀H₂₃NO₄ · HCl 377.86
Morphinan-6-one, 17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-, hydrochloride, (5α)-;
17-(Cyclopropylmethyl)-4,5α-epoxy-3,14-dihydroxy morphinan-6-one hydrochloride CAS RN®: 16676-29-2; UNII: Z6375YW9SF
Free base
C₂₀H₂₃NO₄ 341.41 CAS RN®: 16590-41-3; UNII: 5S6W795CQM
Dihydrate
C₂₀H₂₃NO₄ · HCl · 2H₂O 413.89 CAS RN®: 850808-02-5; UNII: 5P80UKS30B
1 DEFINITION
Naltrexone Hydrochloride contains NLT 98.0% and NMT 102.0% of naltrexone hydrochloride (C₂₀H₂₃NO₄ · HCl), calculated on the anhydrous, solvent-free basis.
2 IDENTIFICATION
Change to read:
2.1 A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A or 197K
Sample: Dissolve 150 mg of Naltrexone Hydrochloride in 25 mL of water in a small separator. Add a few drops of 6 N ammonium hydroxide slowly until no more white precipitate is formed. Extract with three 5-mL portions of chloroform, and pass the extracts through a dry filter, collecting the filtrate in a small flask. Evaporate the filtrate on a steam bath to dryness, and dry the residue at 105° for 1 h.
Acceptance criteria: Meets the requirements
2.2 B. The retention time of the naltrexone peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 Procedure
Solution A: Dissolve about 1.08 g of sodium 1-octanesulfonate and about 23.8 g of sodium acetate in 800 mL of water. Add 1.0 mL of triethylamine and 200 mL of methanol, and adjust with glacial acetic acid to a pH of 6.5 ± 0.1.
Solution B: Dissolve about 1.08 g of sodium 1-octanesulfonate and about 23.8 g of sodium acetate in 400 mL of water. Add 1.0 mL of triethylamine and 600 mL of methanol, and adjust with glacial acetic acid to a pH of 6.5 ± 0.1.
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 35 | 0 | 100 |
| 36 | 100 | 0 |
| 53 | 100 | 0 |
Standard solution: 2.25 mg/mL of USP Naltrexone RS prepared as follows. Transfer an amount of USP Naltrexone RS to a suitable volumetric flask, add 15% of the final volume of methanol and 6% of the final volume of 0.1 N hydrochloric acid, and dissolve by swirling. Dilute with 0.1 M phosphoric acid to volume.
System suitability stock solution: 0.3 mg/mL of USP Naltrexone Related Compound A RS prepared as follows. Transfer a quantity of USP Naltrexone Related Compound A RS to a suitable volumetric flask, add 30% of the final volume of methanol, and dissolve by swirling. Dilute with 0.1 M phosphoric acid to volume.
System suitability solution: 1.125 mg/mL of USP Naltrexone RS and 0.015 mg/mL of USP Naltrexone Related Compound A RS prepared as follows. Transfer suitable volumes of System suitability stock solution and Standard solution to an adequate volumetric flask, and dilute with 0.1 M phosphoric acid to volume.
Sample solution: 2.5 mg/mL of Naltrexone Hydrochloride in 0.1 M phosphoric acid
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: UV 280 nm
- Column: 3.9-mm × 15-cm; 4-µm packing L1
- Flow rate: 1 mL/min
- Injection volume: 20 µL
System suitability
- Samples: Standard solution and System suitability solution
- [Note-The relative retention times for naltrexone and naltrexone related compound A are 1.0 and 1.26, respectively.]
- Suitability requirements
- Resolution: NLT 2.0 between naltrexone and naltrexone related compound A, System suitability solution
- Tailing factor: NMT 1.4, Standard solution
- Relative standard deviation: NMT 0.73%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of naltrexone hydrochloride (C₂₀H₂₃NO₄ · HCl) in the portion of Naltrexone Hydrochloride taken:
Result =(rᵤ/rₛ) × (Cₛ/Cᵤ) × (Mr₁/Mr₂) × 100
rᵤ = peak response of naltrexone from the Sample solution
rₛ = peak response of naltrexone from the Standard solution
Cₛ = concentration of USP Naltrexone RS in the Standard solution (mg/mL)
Cᵤ = concentration of Naltrexone Hydrochloride in the Sample solution (mg/mL)
Mr₁ = molecular weight of naltrexone hydrochloride, 377.86
Mr₂ = molecular weight of naltrexone, 341.41
Acceptance criteria: 98.0%–102.0% on the anhydrous, solvent-free basis
4 IMPURITIES
4.1 Residue on Ignition 〈281〉
NMT 0.1%
4.2 Limit of Total Solvents
The sum of water and total solvents (methanol and Ethanol) is NMT 5.0% for the anhydrous form and NMT 11.0% for the dihydrate form.
4.3 Organic Impurities
Solution A, Solution B, Mobile phase, Standard solution, System suitability stock solution, System suitability solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Sensitivity solution: 2.25 µg/mL of USP Naltrexone RS in 0.1 M phosphoric acid from Standard solution
System suitability
- Samples: Standard solution, System suitability solution, and Sensitivity solution
- Suitability requirements
- Resolution: NLT 2.0 between naltrexone and naltrexone related compound A, System suitability solution
- Tailing factor: NMT 1.4, Standard solution
- Relative standard deviation: NMT 0.73%, Standard solution
- Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Naltrexone Hydrochloride taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × (1/F) × 100
rᵤ = peak response of each impurity from the Sample solution
rₛ = peak response of naltrexone from the Standard solution
Cₛ = concentration of USP Naltrexone RS in the Standard solution (mg/mL)
Cᵤ = concentration of Naltrexone Hydrochloride in the Sample solution (mg/mL)
F = relative response factor (see Table 2)
Acceptance criteria: See Table 2.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Noroxymorphoneᵃ | 0.55 | 1.0 | 0.5 |
| 10-Hydroxynaltrexoneᵇ | 0.70 | 1.0 | 0.5 |
| Naltrexone | 1.00 | 1.0 | - |
| Naltrexone related compound A | 1.26 | 1.0 | 0.5 |
| 2,2′-Bisnaltrexoneᶜ | 1.80 | 2.3 | 0.5 |
| 10-Ketonaltrexoneᵈ | 1.99 | 4.0 | 0.5 |
| Any unspecified impurity | - | 1.0 | 0.5 |
| Total impurities | - | - | 1.5 |
ᵃ 4,5α-Epoxy-3,14-dihydroxymorphinan-6-one.
ᵇ 17-Cyclopropylmethyl-4,5α-epoxy-3,10,14-trihydroxymorphinan-6-one.
ᶜ 17,17′-Bis(cyclopropylmethyl)-4,5α:4′,5′α-diepoxy-3,3′,14,14′-tetrahydroxy-2,2′-bimorphinanyl-6,6′-dione.
ᵈ 17-Cyclopropylmethyl-4,5α-epoxy-3,14-dihydroxymorphinan-6,10-dione.
5 SPECIFIC TESTS
5.1 Content of Chloride
Sample solution: Transfer about 300 mg of Naltrexone Hydrochloride to a 250-mL conical flask, add 50 mL of methanol, 50 mL of water, and 3 mL of nitric acid. Mix to dissolve.
Analysis: Titrate with 0.1 N silver nitrate VS, determining the endpoint potentiometrically. Each milliliter of 0.1 N silver nitrate is equivalent to 3.545 mg of chloride.
Acceptance criteria: 9.20%–9.58%, calculated on the anhydrous, solvent-free basis
5.2 Optical Rotation 〈781S〉, Procedures, Specific Rotation
Sample solution: 25 mg/mL of Naltrexone Hydrochloride in water
Acceptance criteria: −187° to −197°, calculated on the anhydrous, solvent-free basis
5.3 Water Determination 〈921〉, Method I
Determine the water content as directed.
[Note-The result of this test is used in the calculation in the test for Limit of Total Solvents.]
5.4 Clarity of Solution
Sample solution: Dissolve 1.0 g of Naltrexone Hydrochloride in 50 mL of water.
Analysis: Determine the turbidity of the Sample solution (see Nephelometry, Turbidimetry, and Visual Comparison 〈855〉).
Acceptance criteria: NMT 3 NTUs
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
USP Reference Standards 〈11〉
USP Naltrexone RS
USP Naltrexone Related Compound A RS
17-(But-3-en-1-yl)-4,5α-epoxy-3,14-dihydroxymorphinan-6-one hydrochloride.
C₂₀H₂₃NO₄ · HCl 377.86


