Nadolol
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
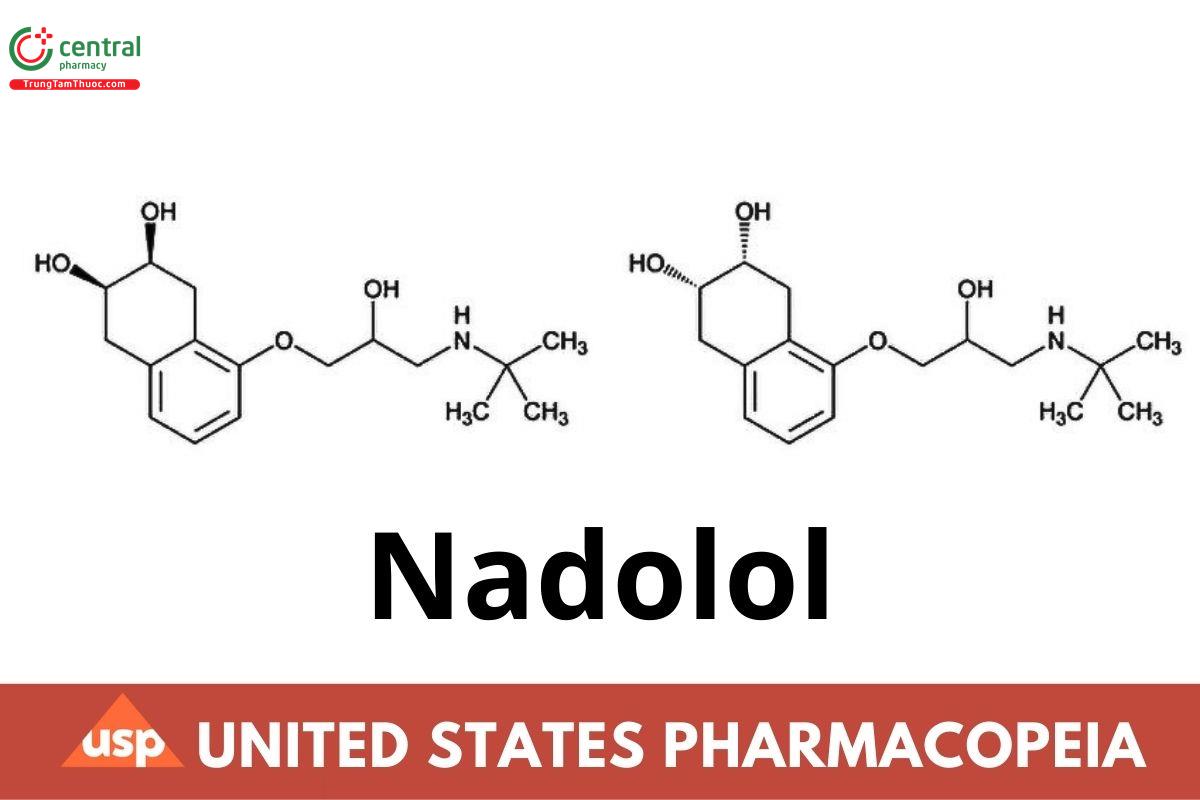
C₁₇H₂₇NO₄ 309.40
2,3-Naphthalenediol, 5-[3-[(1,1-dimethylethyl)amino]-2-hydroxypropoxy]-1,2,3,4-tetrahydro-, cis-;
1-(tert-Butylamino)-3-[(5,6,7,8-tetrahydro-cis-6,7-dihydroxy-1-naphthyl)oxy]-2-propanol;
(2RS,3SR)-5-[3-(tert-Butylamino)-2-hydroxypropoxy]-1,2,3,4-tetrahydronaphthalene-2,3-diol CAS RN®: 42200-33-9; UNII: FEN504330V.
1 DEFINITION
Nadolol contains NLT 98.0% and NMT 102.0% of nadolol (C₁₇H₂₇NO₄), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M or 197A
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Change to read:
3.1 Procedure
Solution A: 0.1% (v/v) trifluoroacetic acid in water prepared as follows. For 1 L, add 1.0 mL of trifluoroacetic acid into 1000 mL of water.
Mobile phase: Acetonitrile and Solution A (15:85)
Standard solution: 0.2 mg/mL of USP Nadolol RS in Mobile phase. Sonication and occasional hand shaking may be necessary for complete dissolution.
Sample solution: 0.2 mg/mL of Nadolol in Mobile phase. Sonication and occasional hand shaking may be necessary for complete dissolution.
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: UV 270 nm
- Column: 4.6-mm × 15-cm; 3.5-µm packing L1
- Column temperature: 30°
- Flow rate: 1 mL/min
- Injection volume: 20 µL
- Run time: NLT 1.7 times the retention time of nadolol
System suitability
- Sample: Standard solution
- Suitability requirements
- Tailing factor: NMT 2.0
- Relative standard deviation: NMT 0.73%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of nadolol (C₁₇H₂₇NO₄) in the portion of Nadolol taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × 100
rᵤ = peak response of nadolol from the Sample solution
rₛ = peak response of nadolol from the Standard solution
Cₛ = concentration of USP Nadolol RS in the Standard solution (mg/mL)
Cᵤ = concentration of Nadolol in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the dried basis
4 OTHER COMPONENTS
Change to read:
4.1 Racemate Composition
Mobile phase: Hexane and alcohol, dehydrated (86:14). For 1 L, add 0.3 mL each of diethylamine and glacial acetic acid into each liter of the mixture.
Standard solution: 0.2 mg/mL of USP Nadolol RS in alcohol, dehydrated
Sample solution: 0.2 mg/mL of Nadolol in alcohol, dehydrated
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: UV 230 nm
- Column: 2.1-mm × 15-cm; 3-µm packing L40
- Flow rate: 0.2 mL/min
- Injection volume: 2 µL
- Run time: NLT 2.4 times the retention time of the last eluted isomer
System suitability
- Sample: Standard solution
- [Note-The relative retention times for the four isomers are 0.48, 0.54, 0.84, and 1.0, respectively. The first and last eluted are isomers of nadolol racemate A while the second and third eluted are isomers of nadolol racemate B.]
- Suitability requirements
- Resolution: NLT 1.0 between the first and the second eluted isomers.
- Relative standard deviation: NMT 5.0% for the ratios between the sum of peak responses of isomers of racemate A and the sum of peak responses of isomers of racemate B.
Analysis
Sample: Sample solution
Calculate the percentage of racemate A in the portion of Nadolol taken:
Result = rᴬ /(rᴬ + rᴮ) × 100
rᴬ = sum of peak responses of isomers of nadolol racemate A
rᴮ = sum of peak responses of isomers of nadolol racemate B
Acceptance criteria: 40%–60%
5 IMPURITIES
5.1 Residue on Ignition 〈281〉: NMT 0.1%
Change to read:
5.2 Organic Impurities
Solution A: Prepare as directed in the Assay.
Solution B: Acetonitrile
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 85 | 15 |
| 3 | 85 | 15 |
| 7 | 50 | 50 |
| 10 | 50 | 50 |
| 10.5 | 85 | 15 |
| 15 | 85 | 15 |
Diluent: Solution B and Solution A (15:85)
System suitability solution: 14 µg/mL of USP Nadolol RS and 1.4 µg/mL each of USP Nadolol Related Compound A RS, USP Nadolol Related Compound B RS, USP Nadolol Related Compound C RS, USP Nadolol Related Compound F RS, and USP Nadolol Related Compound G RS in Diluent
Standard solution: 7 µg/mL each of USP Nadolol RS and USP Nadolol Related Compound F RS in Diluent. Sonication may be necessary for complete dissolution.
Sensitivity solution: 0.35 µg/mL of USP Nadolol RS from the Standard solution in Diluent
Sample solution: 700 µg/mL of Nadolol in Diluent. Sonication and occasional hand shaking may be necessary for complete dissolution.
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: UV 270 nm
- Column: 4.6-mm × 15-cm; 3.5-µm packing L1
- Column temperature: 30°
- Flow rate: 1 mL/min
- Injection volume: 50 µL
System suitability
Samples: System suitability solution, Standard solution, and Sensitivity solution
[Note-The relative retention times in Table 2 are provided as information that could aid in peak assignment.]
Table 2
| Name | Relative Retention Time |
| Nadolol related compound A | 0.78 |
| Nadolol | 1.00 |
| Nadolol related compound Ba | 1.53 |
| Nadolol dimerb | 1.76 |
| Nadolol related compound Ca | 1.83 |
| Nadolol related compound F | 2.03 |
| Nadolol related compound G | 2.10 |
a Multiple peaks may appear to coelute at this relative retention time.
b (2SR,3RS)-5-{3-[tert-Butyl(3-{[(6RS,7SR)-6,7-dihydroxy-5,6,7,8-tetrahydronaphthalen-1-yl]oxy}-2-hydroxypropyl)amino]-2-hydroxypropoxy}-1,2,3,4-tetrahydronaphthalene-2,3-diol] and N,N-Bis{2-hydroxy-3-[(6R,7S)-6,7-dihydroxy-5,6,7,8-tetrahydronaphthalene-1-yloxy]propyl}-N-tert-butylamine.
Suitability requirements
- Resolution: NLT 2.0 between nadolol related compound F and nadolol related compound G, System suitability solution
- Relative standard deviation: NMT 2.0% for both nadolol and nadolol related compound F, Standard solution
- Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of nadolol related compound F in the portion of Nadolol taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × 100
rᵤ = peak response of nadolol related compound F from the Sample solution
rₛ = peak response of nadolol related compound F from the Standard solution
Cₛ = concentration of USP Nadolol Related Compound F RS in the Standard solution (µg/mL)
Cᵤ = concentration of Nadolol in the Sample solution (µg/mL)
Calculate the percentage of nadolol related compound A, nadolol related compound B, nadolol dimer, nadolol related compound C, nadolol related compound G, and any unspecified impurity in the portion of Nadolol taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × (1/F) × 100
rᵤ = peak response of nadolol related compound A, nadolol related compound B, nadolol dimer, nadolol related compound C, nadolol related compound G, or any unspecified impurity from the Sample solution
rₛ = peak response of nadolol from the Standard solution
Cₛ = concentration of USP Nadolol RS in the Standard solution (µg/mL)
Cᵤ = concentration of Nadolol in the Sample solution (µg/mL)
F = relative response factor (see Table 3)
Acceptance criteria: See Table 3. The reporting threshold is 0.05%.
Table 3
| Name | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Nadolol related compound Aa | 1.35 | 0.20 |
| Nadolol related compound Ba | 1.07 | 0.20 |
| Nadolol dimer | 1.11 | 0.20 |
| Nadolol related compound Ca | 1.42 | 0.20 |
| Nadolol related compound F | - | 0.20 |
| Nadolol related compound G | 0.78 | 0.20 |
| Any unspecified impurity | 1.00 | 0.10 |
| Total impurities | - | 0.50 |
a Assessed as the combined peak response of the coeluting peaks.
6 SPECIFIC TESTS
6.1 Loss on Drying 〈731〉
Analysis: Dry under vacuum at 60° for 3 h.
Acceptance criteria: NMT 2.0%
7 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers.
Change to read:
USP Reference Standards 〈11〉
USP Nadolol RS
USP Nadolol Related Compound A RS
(2RS,3SR)-5-(2,3-Dihydroxypropoxy)-1,2,3,4-tetrahydronaphthalene-2,3-diol.
C₁₃H₁₈O₅ 254.28
USP Nadolol Related Compound B RS
(2RS,3SR)-5-(2-Hydroxy-3-methoxypropoxy)-1,2,3,4-tetrahydronaphthalene-2,3-diol.
C₁₄H₂₀O₅ 268.31
USP Nadolol Related Compound C RS
(2SR,3RS)-5-(3-{[(6RS,7SR)-6,7-Dihydroxy-5,6,7,8-tetrahydronaphthalen-1-yl]oxy}-2-hydroxypropoxy)-1,2,3,4-tetrahydronaphthalene-2,3-diol; 1,3-Bis[(6R,7S)-6,7-dihydroxy-5,6,7,8-tetrahydronaphthalene-1-yloxy]propane-2-ol.
C₂₃H₂₈O₇ 416.46
USP Nadolol Related Compound F RS
1-(tert-Butylamino)-3-(naphthalen-1-yloxy)propan-2-ol.
C₁₇H₂₃NO₂ 273.37
USP Nadolol Related Compound G RS
1-(tert-Butylamino)-3-(5,6,7,8-tetrahydronaphthalen-1-yloxy)propan-2-ol.
C₁₇H₂₇NO₂ 277.40


