Mycophenolate Sodium
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
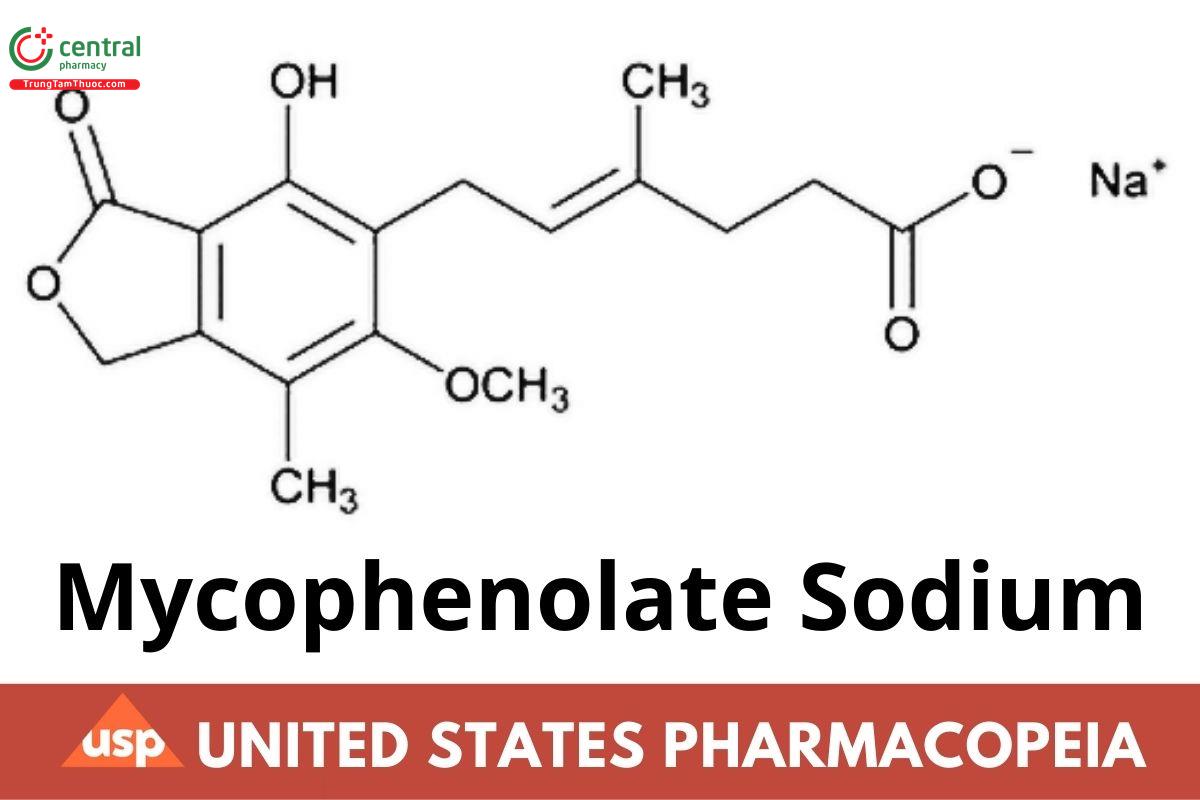
C17H19NaO6 342.32
4-Hexenoic acid, 6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-, monosodium salt, (E)-;Sodium (E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4-enoate
CAS RN: 37415-62-6.
1 DEFINITION
Mycophenolate Sodium contains NLT 98.0% and NMT 102.0% of mycophenolate sodium (C17H19NaO6), calculated on the anhydrous basis.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197A, 197K, or 197M
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Protect solutions from light..
Solution A: Acetonitrile, phosphoric acid, and water (100:0.2:900)
Solution B: Acetonitrile, phosphoric acid, and water (800:0.2:200)
Mobile phase: See Table 1. Return to original conditions, and re-equilibrate the system.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 90 | 10 |
| 35 | 10 | 90 |
| 40 | 10 | 90 |
Diluent: Methanol and water (1:9)
Standard solution: 0.08 mg/mL of USP Mycophenolate Sodium RS in Diluent. Protect from light.
Sample solution: 0.08 mg/mL of Mycophenolate Sodium in Diluent. Protect from light.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 216 nm
Column: 3-mm × 25-cm; 5-µm packing L1
Column temperature: 50°
Flow rate: 0.8 mL/min
Injection volume: 10 µL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 0.73%
Tailing factor: 0.7–1.5
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of mycophenolate sodium (C17H19NaO6) in the portion of Mycophenolate Sodium taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of USP Mycophenolate Sodium RS in the Standard solution (mg/mL)
Cu = concentration of Mycophenolate Sodium in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the anhydrous basis
4 IMPURITIES
ORGANIC IMPURITIES
Protect solutions from light.
Mobile phase, Diluent, Standard solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
System suitability stock solution 1: 0.02 mg/mL of USP Mycophenolate Mofetil Related Compound B.RS prepared as follows. Transfer USP Mycophenolate Mofetil Related Compound B RS to a suitable volumetric flask and dilute in methanol equivalent to 10% of the final volume, and then dilute with water to volume.
System suitability stock solution 2: Transfer 4.0 mL of System suitability stock solution 1 to a 100-mL volumetric flask and dilute with Diluent to volume.
System suitability solution: 0.8 µg/mL of USP Mycophenolate Mofetil Related Compound B RS and 0.08 mg/mL of USP Mycophenolate Sodium RS prepared by dissolving a suitable amount of USP Mycophenolate Sodium RS in System suitability stock solution 2
Sensitivity solution: 0.024 µg/mL of USP Mycophenolate Sodium RS in Diluent from Standard solution
System suitability
Samples: Standard solution, System suitability solution, and Sensitivity solution
Suitability requirements
Resolution: NLT 1.5 between the mycophenolate mofetil related compound B and mycophenolate peaks, System suitability solution
Relative standard deviation: NMT 0.73%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Mycophenolate Sodium taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response of each impurity from the Sample solution
rs = peak response of mycophenolate from the Standard solution
Cs = concentration of USP Mycophenolate Sodium RS in the Standard solution (mg/mL)
Cu = concentration of Mycophenolate Sodium in the Sample solution (mg/mL)
Acceptance criteria: See Table 2. Disregard any impurity peak less than 0.05%.
Table 2
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Mycophenolate mofetil related compound Ba | 0.9 | 0.1 |
| Mycophenolate | 1.0 | - |
| Any individual unspecied impurity | - | 0.07 |
| Total impurities | - | 0.4 |
a (RS)-7-Hydroxy-5-methoxy-4-methyl-6-[2-(5-methyl-2-oxo-tetrahydrofuran-5-yl)ethyl]-3H-isobenzofuran-1-one.
5 SPECIFIC TESTS
SODIUM CONTENT
Standard stock solution: Use commercially available sodium atomic absorption spectroscopy standard solution of 1000 µg/mL of sodium in 0.5 M nitric acid.
Diluent: Transfer 3 mL of nitric acid to a 250-mL volumetric flask, and dilute with water to volume.
Standard solution A: 6.0 µg/mL of sodium in Diluent from Standard stock solution
Standard solution B: 9.0 µg/mL of sodium in Diluent from Standard stock solution
Standard solution C: 12.0 µg/mL of sodium in Diluent from Standard stock solution
Sample solution: 0.14 mg/mL of Mycophenolate Sodium prepared as follows. Weigh 30-40 mg of Mycophenolate Sodium into a digestion vessel, add 3 mL of nitric acid and digest at 150° for 5 h. Allow to cool, transfer the digestion solution to a 250-mL volumetric flask, and dilute with water to volume.
Instrumental conditions
(See Atomic Absorption Spectroscopy (852).)
Mode: Atomic absorption spectrophotometry
Analytical wavelength: 589.0 nm
Lamp: Sodium hollow-cathode
Flame: Air-acetylene
Blank: Diluent
System suitability
Sample: Standard solution B
Suitability requirements
Relative standard deviation: NMT 5% for absorbance from three readings
Analysis
Samples: Standard solutions, Sample solution, and Blank
Plot the absorbances of the Blank and Standard solutions versus their concentrations of sodium (0, 6.0, 9.0, and 12.0 µg/mL), and draw a calibration curve best fitting the four points. From the graph so obtained, determine the concentration, in µg/ml, of sodium in the Sample solution.
Calculate the percentage of sodium in the portion of Mycophenolate Sodium taken.
Result = F × (Cs /Cu ) × 100
F = conversion factor (0.001 mg/µg)
Cs = concentration of sodium in the Sample solution (µg/mL)
Cu = concentration of Mycophenolate Sodium in the Sample solution (mg/mL)
Acceptance criteria: 5.7%–7.7% on the anhydrous basis
Change to read:
X-RAY POWDER DIFFRACTION (941) (CN 1-MAY-2022): Its X-ray diffraction pattern conforms to that of USP Mycophenolate Sodium RS. similarly determined.
WATER DETERMINATION, Method la(921): NMT 1.5%
MICROBIAL ENUMERATION TESTS (61) and TESTS FOR SPECIFIED MICROORGANISMS (62): The total aerobic microbial limit does not exceed 103 cfu/g. The total yeasts and molds count does not exceed 102 cfu/g.
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers. Protect from light.
USP Reference Standards 〈11〉
USP Mycophenolate Mofetil Related Compound B RS
(RS)-7-Hydroxy-5-methoxy-4-methyl-6-[2-(5-methyl-2-oxo-tetrahydrofuran-5-yl)ethyl]-3H-isobenzofuran-1-one.
C17H20O6 320.34
USP Mycophenolate Sodium RS


