Moxifloxacin Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
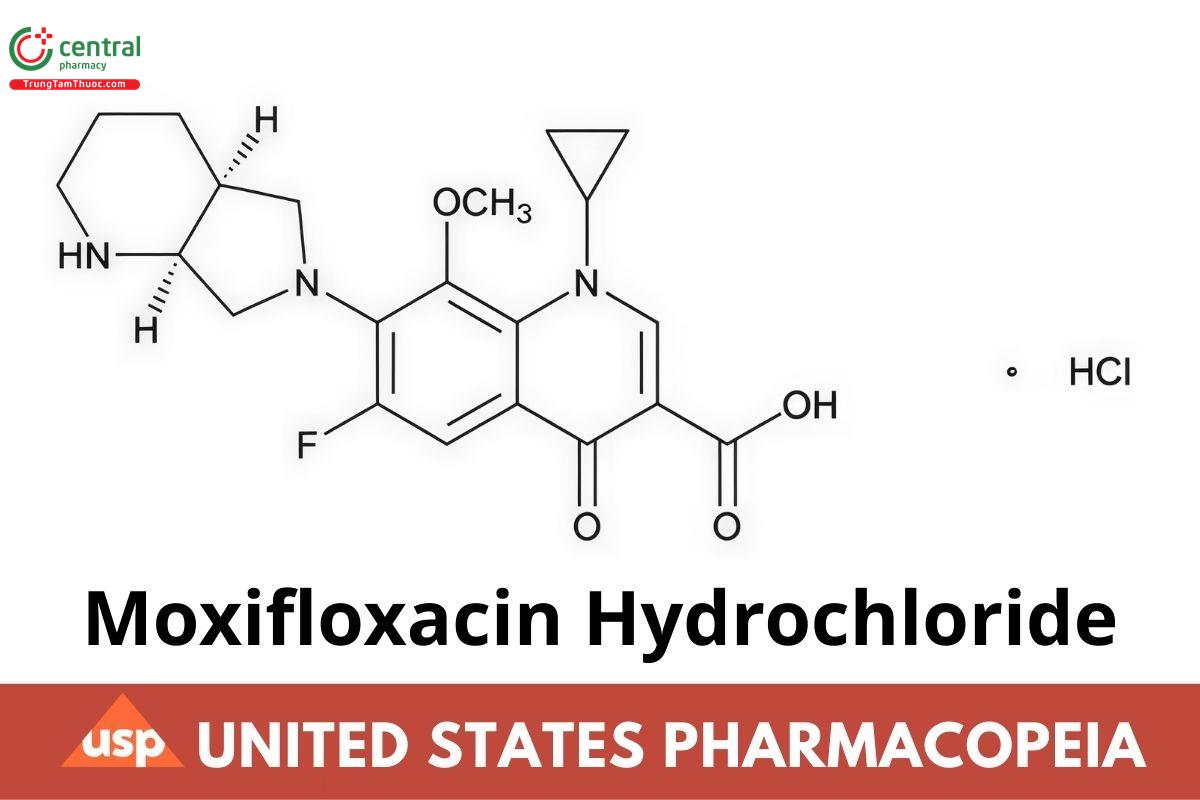
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C21H24FN3O4 · HCl 437.89
(4aS-cis)-1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl)-4-oxo-3-quinolinecarboxylic acid, monohydrochloride;
1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-3-quinolinecarboxylic acid, monohydrochloride CAS RN®: 186826-86-8; UNII: C53598599T.
1 DEFINITION
Moxifloxacin Hydrochloride contains NLT 98.0% and NMT 102.0% of moxifloxacin hydrochloride (C21H24FN3O4 · HCl), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. ▲SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K ▲(CN 1-MAY-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
C. IDENTIFICATION TESTS-GENERAL (191), Chloride
Sample solution: To a solution (1 in 160) add diluted nitric acid, and filter.
Acceptance criteria: Meets the requirements
3 ASSAY
PROCEDURE
Buffer: Dissolve 0.5 g of tetrabutylammonium hydrogen sulfate and 1.0 g of monobasic potassium phosphate in water, add 2 mL of phosphoric acid, dilute with water to 1000 ml, mix, and pass through a filter of 0.45-µm pore size.
Mobile phase: Methanol and Buffer (28:72)
Diluent: Add 20 mg of anhydrous sodium sulfite to 1000 mL of Buffer, mix gently, and pass through a filter of 0.45-µm pore size.
System suitability solution: 0.1 mg/mL of USP Moxifloxacin Hydrochloride RS and 1 µg/mL of USP Moxifloxacin Related Compound A RS, in Diluent
Standard solution: 0.1 mg/mL of USP Moxifloxacin Hydrochloride RS in Diluent
Sample solution: 0.1 mg/mL of Moxifloxacin Hydrochloride in Diluent
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 293 nm
Column: 4.0-mm x 25-cm; 5-µm packing L11
Column temperature: 45°
Flow rate: 0.9 mL/min
Injection volume: 25 µL
System suitability
Samples: System suitability solution and Standard solution
[NOTE-The relative retention times for moxifloxacin and moxifloxacin related compound A are about 1.0 and 1.2, respectively.]
Suitability requirements
Resolution: NLT 1.5 between moxifloxacin and moxifloxacin related compound A, System suitability solution
Tailing factor: NMT 2.0, Standard solution
Relative standard deviation: NMT 0.73%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of moxifloxacin hydrochloride (C21H24FN3O4 · HCl) in the portion of Moxifloxacin Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Moxifloxacin Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Moxifloxacin Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 98.0%~102.0% on the anhydrous basis
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281)
NMT 0.1%
4.2 CHLORIDE AND SULFATE (221), Sulfate
Sample: 0.6 g
Acceptance criteria: 0.04%; the Sample shows no more sulfate than corresponds to 0.25 mL of 0.020 N sulfuric acid.
4.3 ORGANIC IMPURITIES
Protect all solutions containing moxifloxacin from light.
Mobile phase, Diluent, System suitability solution, and Sample solution: Prepare as directed in the Assay.
Standard solution: 2 µg/mL of USP Moxifloxacin Hydrochloride RS in Diluent
Sensitivity solution: 0.05 µg/mL of USP Moxifloxacin Hydrochloride RS from the Standard solution in Diluent. Store the Sensitivity solution under refrigeration and protected from light.
Chromatographic system: Proceed as directed in the Assay with a run time of two times the retention time of moxifloxacin.
System suitability
Samples: System suitability solution, Standard solution, and Sensitivity solution
Suitability requirements
Resolution: NLT 1.5 between moxifloxacin and moxifloxacin related compound A, System suitability solution
Tailing factor: NMT 2.0, Standard solution
Relative standard deviation: NMT 2.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Sample solution and Standard solution
Calculate the percentage of each impurity in the portion of Moxifloxacin Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of moxifloxacin from the Standard solution s
CS = concentration of USP Moxifloxacin Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Moxifloxacin Hydrochloride in the Sample solution (mg/mL)
F = relative response factor (see Table 1)
Acceptance criteria: See Table 1.
Table 1
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Moxifloxacin | 1.0 | — | — |
| Moxifloxacin related compound Aa | 1.15 | 1.0 | 0.1 |
| Moxifloxacin related compound Bb | 1.32 | 0.71 | 0.1 |
| Moxifloxacin related compound Cc | 1.48 | 1.0 | 0.1 |
| Moxifloxacin related compound Dd | 1.71 | 1.0 | 0.1 |
| Moxifloxacin related compound Ee | 1.83 | 0.29 | 0.1 |
| Other individual impurity | — | 1.0 | 0.1 |
| Total impurities | — | — | 0.5 |
a 1-Cyclopropyl-6,8-difluoro-1,4-dihydro-7-[(4aS, 7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-3-quinolinecarboxylic acid.
b 1-Cyclopropyl-6,8-dimethoxy-1,4-dihydro-7-[(4aS, 7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-3-quinolinecarboxylic acid.
c 1-Cyclopropyl-8-ethoxy-6-fluoro-1,4-dihydro-7-[(4aS, 7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-3-quinolinecarboxylic acid.
d 1-Cyclopropyl-8-fluoro-6-methoxy-1,4-dihydro-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-3-quinolinecarboxylic acid.
e1-Cyclopropyl-6-fluoro-8-hydroxy-1,4-dihydro-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-3-quinolinecarboxylic acid.
4.4 ENANTIOMERIC PURITY
Protect all solutions containing moxifloxacin from light.
Buffer: 0.47 g/L of anhydrous cupric sulfate and 1.31 g/L of isoleucine in water. Adjust with 0.1 N sodium hydroxide to a pH of 4.50. AL
Solution A: Methanol and Buffer (500:1500)
Solution B: Methanol and Buffer (225:450)
Mobile phase: See Table 2.
Table 2
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 50 | 100 | 0 |
| 51 | 0 | 100 |
| 61 | 0 | 100 |
| 62 | 100 | 0 |
| 85 | 100 | 0 |
System suitability solution: 8 mg/mL of USP Moxifloxacin Hydrochloride RS and 8 µg/mL of USP Moxifloxacin Related Compound G RS in Solution A
Sensitivity solution: 0.8 µg/mL of USP Moxifloxacin Related Compound G RS in Solution A
Sample solution: 8 mg/mL of Moxifloxacin Hydrochloride in Solution A
Chromatographic system
(See Chromatography (621). System Suitability.)
Mode: LC
Detector: UV 295 nm
Column: 3.0-mm x 15-cm; 3-µm packing L1
Flow rate: 0.42 mL/min
Injection volume: 1.5 µL
System suitability
Samples: System suitability solution and Sensitivity solution
[NOTE-The relative retention times for moxifloxacin related compound G and moxifloxacin are 0.78 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.0 between moxifloxacin related compound G and moxifloxacin, System suitability solution
Signal-to-noise ratio: NLT 5, Sensitivity solution
Analysis
Sample: Sample solution
Calculate the percentage of moxifloxacin related compound G in the portion of Moxifloxacin Hydrochloride taken:
Result = [rU(rU/rS) ×] x 100
rU = peak response of moxifloxacin related compound G from the Sample solution
rS = peak response of moxifloxacin from the Sample solution
Acceptance criteria: NMT 0.15%
5 SPECIFIC TESTS
5.1 MICROBIAL ENUMERATION TESTS (61) and TESTS FOR SPECIFIED MICROORGANISMS (62)
Total aerobic microbial count: NMT 103 cfu/g
Total combined molds and yeasts count: NMT 102 cfu/g
5.2 PH (791)
Sample solution: 2 mg/mL
Acceptance criteria: 3.9-4.6
5.3 WATER DETERMINATION (921), Method I. Method la
NMT 4.5%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight, light-resistant containers. Store at room temperature.
USP REFERENCE STANDARDS (11)
USP Moxifloxacin Hydrochloride RS
USP Moxifloxacin Related Compound A RS
1-Cyclopropyl-6,8-difluoro-1,4-dihydro-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-3-quinolinecarboxylic acid.
C20H21F2N3O3 389.40
USP Moxifloxacin Related Compound G.RS
1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aR,7aR)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-3-quinolinecarboxylic acid, monohydrochloride.
C21H24FN3O4 · HCl 437.89


