Moxidectin
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
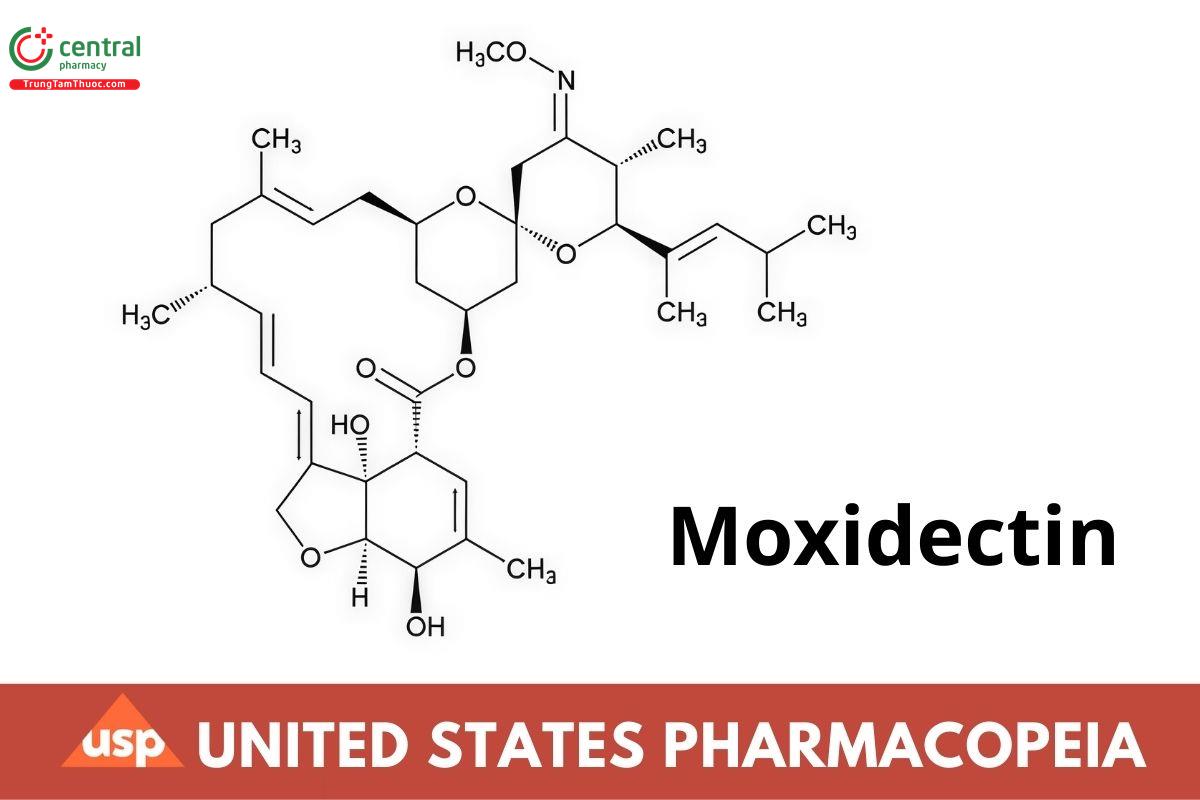
C37H53NO8 639.82
(6R,25S)-5-O-Demethyl-28-deoxy-25-[(E)-1,3-dimethyl-1-butenyl]-6,28-epoxy-23-oxomilbemycin B 23-(E)-(O-methyloxime);
(2aE,4E,5'R,6R,6'S,8E, 11R,13S, 15S, 17aR,20R,20aR,20bS)-6'-[(E)-1,3-Dimethyl-1-butenyl]-5,6,6,7,10,11,14,15,17,20,20a,20b-dodecahydro-20,20b-dihydroxy-5',6,8,19-tetramethylspiro (11,15-methano-2H,13H,17H-furo [4,3,2-pq][2,6]benzodioxacyclooctadecin-13,2'-[2H]pyran]-4',17(3'H)-dione 4'-(E)-(0-methyloxime) CAS RN®: 113507-06-5; UNII: NGU5H31Y09.
1 DEFINITION
Moxidectin contains NLT 92.0% and NMT 102.0% of moxidectin (C37H53NO8), calculated on the anhydrous basis. It may contain a suitable antioxidant.
2 IDENTIFICATION
Change to read:
A. ▲SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K ▲(CN 1-MAY-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Buffer: Dissolve 7.7 g of ammonium acetate in 400 mL of water, and adjust with glacial acetic acid to a pH of 4.8.
Mobile phase: Acetonitrile and Buffer (60:40)
Standard solution: 1.0 mg/mL of USP Moxidectin RS in acetonitrile. Sonicate if necessary to facilitate dissolution.
Sample solution: 1.0 mg/mL of Moxidectin in acetonitrile. Sonicate if necessary to facilitate dissolution.
Chromatographic system
(See Chromatography (621), System Suitability)
Mode: LC
Detector: UV 242 nm
Column: 3.9-mm x 15-cm; 4-µm packing L1
Column temperature: 50°
Flow rate: 2.5 mL/min
Injection volume: 10 µL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 1%, for 4 replicate injections
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of moxidectin (C37H53NO8) in the portion of Moxidectin taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Moxidectin RS in the Standard solution (mg/mL)
CU = concentration of Moxidectin in the Sample solution (mg/mL)
Acceptance criteria: 92.0%-102.0% on the anhydrous basis
4 IMPURITIES
RESIDUE ON IGNITION (281): NMT 0.2%
Delete the following:
HEAVY METALS, Method II (231): NMT 20 ppm ▲(Official 1-Jan-2018)
4.1 ORGANIC IMPURITIES: EARLY-ELUTING IMPURITIES
Buffer, Mobile phase, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
System suitability solution: 1.0 mg/mL of USP Moxidectin System Suitability Mixture RS in acetonitrile. Sonicate if necessary to facilitate dissolution.
Standard solution: 0.01 mg/mL of Moxidectin in acetonitrile from the Sample solution
System suitability
Sample: System suitability solution
Suitability requirements
Peak-to-valley ratio: NLT 3.0 between moxidectin 17a-epimer and moxidectin
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each early-eluting impurity in the portion of Moxidectin taken:
Result = (rU/rS) x F x D x 100
rU = peak response of each early-eluting impurity from the Sample solution
rS = peak response of moxidectin from the Standard solution
F = Assay value expressed as a decimal
D= dilution factor used to prepare the Standard solution, 0.01
Acceptance criteria: See Table 1.
The reporting level for impurities is 0.1%. Disregard the peak due to the stabilizer (identify this peak, where applicable, by injecting a suitable reference solution).
Table 1
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Moxidectin butenyl analoga | 0.5 | 1.5 |
| 5-Demethyl moxidectinb | 0.7 | 0.5 |
| Moxidectin pentenyl analogc | 0.75 | 1.5 |
| Moxidectin 17a-epimerd | 0.9 | 2.5 |
| Moxidectin | 1.0 | — |
| Sum of moxidectin 19-S-17a-enee and moxidectin ethyl isomersf | 1.3–1.5 | 1.7g |
| Milbemycin B analog (moxidectin open ring)h | 1.6 | 1.5 |
| Any other individual impurity eluting before milbemycin B analog (moxidectin open ring) | — | 0.5 |
a (2aE,4E,5'R,6R, 6'S,8E, 11R,13S, 15S, 17aR, 20R,20aR,20bS)-6'-[(E)-But-2-en-2-yl]-5',6,6',7,10,11,14,15,17a,20,20a, 20b-dodecahydro-20,20b-dihydroxy-5',6,8,19-tetramethylspiro [11,15-methano-2H, 13H,17H-furo [4,3,2-pq][2,6] benzodioxacyclooctadecin-13,2'-[2H]pyran]-4',17(3'H)-dione 4'-(E)-(O-methyloxime).
b (2aE,4E,5'R,6R,6'S,8E, 11R,13S, 15S, 17aR,20R,20aR,20bS)-5',6,6',7,10,11,14,15,17a,20,20a, 20b-Dodecahydro-20,20b-dihydroxy-6'-[(E)-4-methylpent-2-en-2-yl]-6,8,19-trimethylspiro [11,15-methano-2H, 13H,17H-furo [4,3,2-pq][2,6] benzodioxacyclooctadecin-13,2'-[2H]pyran]-4',17(3'H)-dione 4'-(E)-(O-methyloxime).
c (2aE,4E,5'R,6R,6'S,8E, 11R,13S, 15S, 17aR,20R,20aR,20bS)-5',6,6,7,10,11,14,15,17,20,20a, 20b-Dodecahydro-20,20b-dihydroxy-5,6,8,19-tetramethyl-6'-[(E)-pent-2-en-2-yl]spiro [11,15-methano-2H, 13H,17H-furo [4,3,2-pq][2,6] benzodioxacyclooctadecin-13,2'-[2H]pyran]-4',17(3'H)-dione 4'-(E)-(O-methyloxime).
d (2aE,4E,5'R,6R,6'S,8E,11R,135, 155, 17aS,20R,20aR,20bS)-5',6,6',7,10,11,14,15,17a,20,20a, 20b-Dodecahydro-20,20b-dihydroxy-6'-[(E)-4-methylpent-2-en-2-yl)-5',6,8,19-tetramethylspiro (11,15-methano-2H, 13H,17H-furo[4,3,2-pq][2,6] benzodioxacyclooctadecin-13,2'-[2H]pyran]-4',17(3'H)-dione 4'-(E)-(O-methyloxime).
e (2aE,4E,5'R,6R,6'S,8E, 11R,13S, 15S,19S,20R,20aR,20bS)-5',6,6',7,10,11,14,15,19,20,20a,20b-Dodecahydro-20,20b-dihydroxy-6'-[(E)-4-methylpent-2-en-2-yl]-5,6,8,19-tetramethylspiro [11,15-methano-2H,13H,17H-furo [4,3,2-pq][2,6]benzodioxacyclooctadecin-13,2'-[2]ругап]-4',17(3'H)-dione 4'-(E)-(O-methyloxime).
f Mixture of five possible isomers, where one methyl group in the analyte is replaced with an ethyl group.
g If present, moxidectin 19-S-17a-ene and the moxidectin ethyl isomers may not be completely resolved by the method. These peaks are integrated together to determine conformance.
h (2'R,35,5'S,6'S,7R,9E, 12R, 13E,15E, 16aS, 18S,20aR)-16a, 18-Dihydroxy-5',10,12,16,19-pentamethyl-6'-[(E)-4-methylpent-2-en-2-yl]-3,4, 5,6,7,8,11,12,16,17,18,20a-dodecahydro-1H-spiro[3,7-methanobenzo[g][1,5]dioxacyclooctadecin-5,2'-[2H]pyran]-14'-dione (E)-(O-methyloxime).
4.2 ORGANIC IMPURITIES: LATE-ELUTING IMPURITIES
Buffer: Dissolve 3.8 g of ammonium acetate in 250 mL of water, and adjust with glacial acetic acid to a pH of 4.2.
Mobile phase: Acetonitrile and Buffer (75:25)
System suitability solution: 3.0 mg/mL of USP Moxidectin System Suitability Mixture RS in acetonitrile. Sonicate if necessary to facilitate dissolution.
Sample solution: 3.0 mg/mL of Moxidectin in acetonitrile. Sonicate if necessary to facilitate dissolution.
Standard solution: 0.03 mg/mL of Moxidectin in acetonitrile from the Sample solution
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 242 nm
Column: 3.9-mm x 15-cm; 4-um packing L1
Column temperature: 35°
Flow rate: 2 mL/min
Injection volume: 10 µL
Run time: NLT 10 times the retention time of moxidectin
System suitability
Sample: System suitability solution
Suitability requirements
Resolution: NLT 1.0 between moxidectin deoxydiene/methylthiomethoxymoxidectin and 20b-methylthiomoxidectin
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each late-eluting impurity in the portion of Moxidectin taken:
Result = (rU/rS) x Fx D x 100
rU = peak response of each late-eluting impurity from the Sample solution
rS = peak response of moxidectin from the Standard solution
F = Assay value expressed as a decimal
D = dilution factor used to prepare the Standard solution, 0.01
Acceptance criteria: See Table 2.
The reporting level for impurities is 0.1%. Disregard the peak due to the stabilizer (identify this peak, where applicable, by injecting a suitable reference solution).
Table 2
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Moxidectin | 1.0 | — |
| Moxidectin deoxydienea and 4'-methylthiomethoxymoxidectinb | 2.0 | 1.0c |
| 20b-Methylthiomoxidectind | 2.2 | 0.5 |
| 20-Nitrobenzoylmoxidectine | 3.4 | 0.5 |
| Any other individual impurity eluting after the milbemycin B analog (moxidectin open ring) (1.4 RRT) | — | 0.5 |
a (2aE,4E,5'R,6R,6'S,8E, 11R,13S, 15S,20aR,20bS)-5',6,6',7,10,11,14,15,20a,20b-Decahydro-20b-hydroxy-6'-[(E)-4-methylpent-2-en-2-yl]-5',6,8,19-tetramethylspiro [11,15-methano-2H, 13H,17H-furo [4,3,2-pq][2,6]benzodioxacyclooctadecin-13,2'-[2H]pyran]-4',17(3'H)-dione 4'-(E)-(O-methyloxime).
b (2aE,4E,4'S,5 R,6R, 6'S,8E, 11R,13S, 15S,17aR,20R,20aR,20bS)- 3',4',5',6,6',7,10,11,14,15,17,20,20a,20b-Tetradecahydro-20,20b-dihydroxy-6'-[(E)-4-methylpent-2-en-2-yl]-4'-methylthiomethoxy-5',6,8,19-tetramethylspiro [11,15-methano-2H, 13H,17H-furo[4,3,2-pq][ 2,6] benzodioxacyclooctadecin-13,2'-[2H]pyran]-17-one.
c If present, impurities moxidectin deoxydiene and 4-methylthiomethoxymoxidectin may not be completely resolved by the method. These peaks are integrated together to determine conformance.
d (2aE,4E,5'R,6R,6'S,8E, 11R,13S, 15S, 17aR,20R,20aR,20bS)-5',6,6',7,10,11,14,15,17,20,20a,20b-Dodecahydro-20-hydroxy-6'-[(E)-4-methylpent-2-en-2-yl]-20b-methylthiomethoxy-5',6,8,19-tetramethylspiro [11,15-methano-2H,13H,17H-furo[4,3,2-pq][2,6]benzodioxacyclooctadecin-13,2'-[2H]pyran]-4',17(3'H)-dione 4'-(E)-(O-methyloxime).
e (2aE,4E,5'R,6R,6'S,8E, 11R,135,155,17aR,20R,20aR,20bS)-5',6,6',7,10,11,14,15,17,20,20a,20b-Dodecahydro-20b-hydroxy-6'-[(E)-4-methylpent-2-en-2-yl]-20-(4-nitrobenzoyloxy)-5',6,8,19-tetramethylspiro [11,15-methano-2H,13H,17H-furo[4,3,2-pq] [2,6] benzodioxacyclooctadecin-13,2'-[2H]pyran]-4',17(3'H)-dione 4'-(E)-(O-methyloxime).
4.3 TOTAL ORGANIC IMPURITIES
Analysis: Calculate the sum of all impurities found in the tests for Organic Impurities: Early-Eluting Impurities and Organic Impurities: Late-Eluting Impurities in the portion of Moxidectin taken.
Acceptance criteria: NMT 7.0%
5 SPECIFIC TESTS
WATER DETERMINATION (921), Method : NMT 1.3%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed, light-resistant containers, and store in a refrigerator.
Change to read:
LABELING: If it is intended for use in animals, it is so labeled (RB 27-Jun-2018) Label it to state the name(s) and amount(s) of any added substance(s).
USP REFERENCE STANDARDS (11)
USP Moxidectin RS
USP Moxidectin System Suitability Mixture RS


