Montelukast Sodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
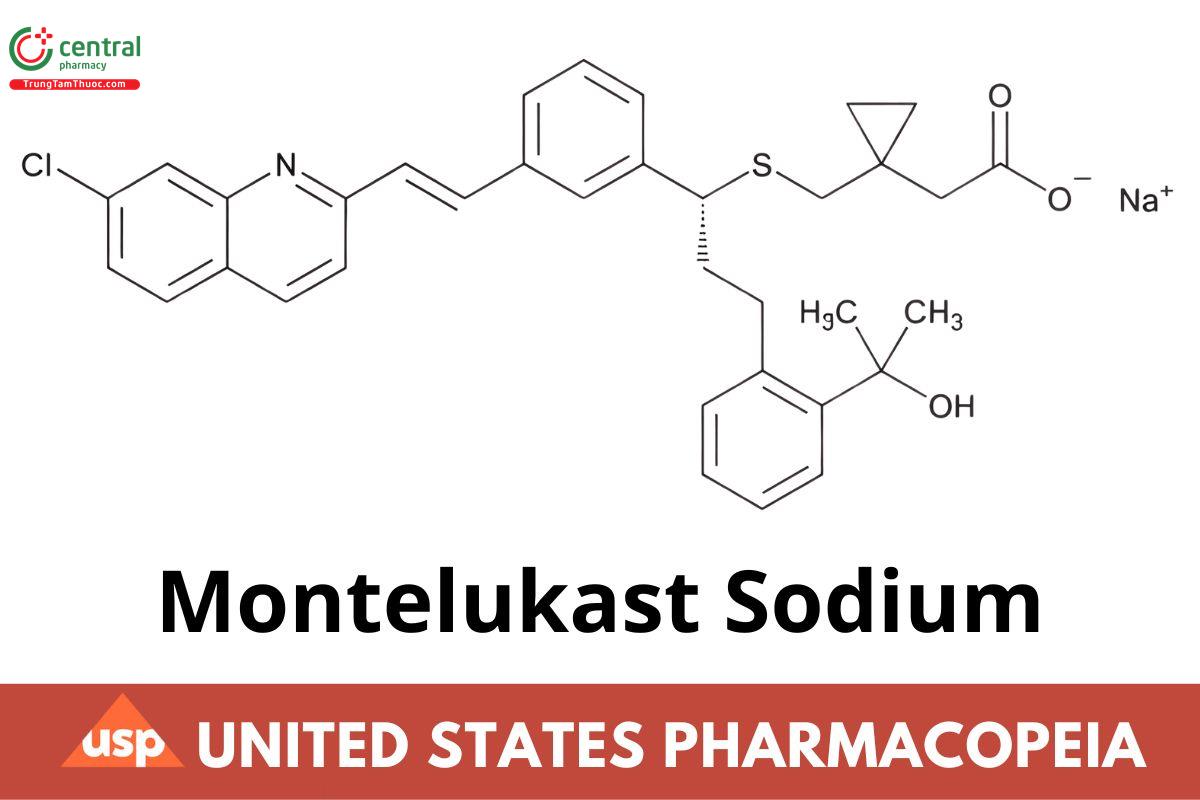
C35H35CINNaO3S 608.17
Cyclopropaneacetic acid, 1-[[[1-[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]-, sodium salt, [R-,(E)]-;
Sodium 1-[[[(R)-m-[(E)-2-(7-chloro-2-quinolyl) vinyl]-a-[o-(1-hydroxy-1-methylethyl)phenethyl benzyl thio]-methyl]cyclopropaneacetate CAS RN®: 151767-02-1; UNII: U103J18SFL.
C35H35CINNO3S 586.18
Montelukast CAS RN®: 158966-92-8; UNII: MHM278SD3E.
1 DEFINITION
Montelukast Sodium contains NLT 98.0% and NMT 102.0% of CH 35 35 anhydrous and solvent-free basis.
2 IDENTIFICATION
Change to read:
A. ▲SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 1974, 197K, or 197MA ▲(CN 1-May-2020)
B. IDENTIFICATION TESTS GENERAL, Sodium (191)
Sample: 100 mg
Analysis: Ignite the Sample in a crucible until an almost white residue is obtained. Take up the residue in 2 mL of water, and filter.
Acceptance criteria: The filtrate meets the requirements of test A.
C. Meets the requirements of the test for Enantiomeric Purity.
3 ASSAY
[NOTE-Avoid exposure of the samples to light. Use low-actinic glassware.]
PROCEDURE
Solution A: Add 1.5 mL of trifluoroacetic acid to 1 L of water.
Solution B: Add 1.5 mL of trifluoroacetic acid to 1 L of acetonitrile.
Mobile phase: See Table 1. Return to original conditions and re-equilibrate the column.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 60 | 40 |
| 3.0 | 60 | 40 |
| 16.0 | 49 | 51 |
Diluent: Methanol and water (9:1)
Standard solution: 0.13 mg/mL of USP Montelukast Dicyclohexylamine RS in Diluent
Sample solution: 0.1 mg/mL of Montelukast Sodium in Diluent
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 238 nm
Column: 4.6-mm x 5-cm; 1.8-µm packing L11
Column temperature: 30°
Flow rate: 1.2 mL/min
Injection size: 10 µL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 0.73%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of montelukast sodium (C35H35CINNaO3S) in the portion of Montelukast Sodium taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) ×100
rU = peak area from the Sample solution
rS = peak area from the Standard solution
CS = concentration of the Standard solution (mg/mL)
CU = concentration of the Sample solution (mg/mL)
Mr1 = molecular weight of montelukast sodium, 608.17
Mr2 = molecular weight of montelukast dicyclohexylamine, 767.50
Acceptance criteria: 98.0%-102.0% on the anhydrous and solvent-free basis
4 IMPURITIES
4.1 ORGANIC IMPURITIES
[NOTE-Avoid exposure of the samples to light. Use low-actinic glassware.]
Solution A, Solution B, Mobile phase, Diluent, and Chromatographic system: Proceed as directed in the Assay.
Impurity solution: 1 mg/mL of USP Montelukast for Peak Identification RS in Diluent
System suitability solution: Transfer 1 mL of the Impurity solution to a colorless glass vial, and expose to ambient light for approximately 20 min to generate the cis-isomer of montelukast.
Sample solution: 1 mg/mL of Montelukast Sodium in Diluent
Sensitivity solution: 0.5 µg/mL of Montelukast Sodium in Diluent from the Sample solution
System suitability
Samples: System suitability solution and Sensitivity solution
Suitability requirements
Resolution: NLT 2.5 between the cis-isomer and montelukast; NLT 1.5 between montelukast and the methylketone impurity, System suitability solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Montelukast Sodium taken:
Result = (rU/rT) x 100
rU = peak response of each impurity from the Sample solution
rT = sum of all the peak responses from the Sample solution
Acceptance criteria: See Table 2.
Reporting level for impurities: 0.05%
Table 2
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Sulfoxide impuritya | 0.4 0.2 | 0.2 |
| Cis-isomerb | 0.8 | 0.15 |
| Michael Adducts 1c and 2d | 0.9 | 0.15* |
| Montelukast | 1.0 | — |
| Methylketone impuritye | 1.2 | 0.15 |
| Methylstyrene impurityf | 1.9 | 0.3 |
| Any other individual impurity | — | 0.10 |
| Total impurities | — | 0.6 |
* These two impurities are not resolved by the method and need to be integrated together to determine conformance.
a [1-[[[1-[3-[(E)-2-(7-Chloroquinolin-2-yl)ethenyl)phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl sulfinyl]methyl]cyclopropyl acetic acid.
b [1-[[[(1R)-1-[3-[(Z)-2-(7-Chloroquinolin-2-yl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl sulfanyl]methyl]cyclopropyl acetic acid.
c 1-[[[(1R)-1-[3-[(1R)-1-[[[1-(Carboxymethyl)cyclopropyl]methyl]sulfanyl]-2-(7-chloroquinolin-2-yl)ethyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]sulfanyl]methyl]cyclopropyl acetic acid.
d 1-[[[(1R)-1-[3-[(1S)-1-[[[1-(Carboxymethyl)cyclopropyl]methyl]sulfanyl]-2-(7-chloroquinolin-2-yl)ethyl)phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]sulfanyl]methyl]cyclopropyl acetic acid.
e [1-[[[(1R)-3-(2-Acetylphenyl)-1-[3-[(E)-2-(7-chloroquinolin-2-yl)ethenyl)phenyl]propyl sulfanyl]methyl]cyclopropyl acetic acid.
f [1-[[[(1R)-1-[3-[(E)-2-(7-Chloroquinolin-2-yl)ethenyl]phenyl]-3-[2-(1-methylethenyl)phenyl]propyl]sulfanyl]methyl]cyclopropyl]acetic acid.
4.2 ENANTIOMERIC PURITY
[NOTE-Avoid exposure of the samples to light. Use low-actinic glassware.]
Solution A: 2.3 g/L of ammonium acetate in water. Adjust with glacial acetic acid to a pH of 5.7.
Solution B: Methanol and acetonitrile (60:40)
Mobile phase: See Table 3.
Table 3
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 70 | 30 |
| 30 | 60 | 40 |
| 35 | 60 | 40 |
Diluent: Acetonitrile and water (1:1)
System suitability solution: 0.1 mg/mL of USP Montelukast Racemate RS in Diluent
Sample solution: 1 mg/mL of Montelukast Sodium in Diluent
Sensitivity solution: 1 µg/mL of Montelukast Sodium in Diluent from the Sample solution
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.0-mm x 15-cm; 5-µm packing L41
Column temperature: 30°
Flow rate: 0.9 mL/min
Injection size: 10 µL
System suitability
Samples: System suitability solution and Sensitivity solution
[NOTE-The relative retention times are 1.0 for montelukast, which is the R-enantiomer, and 0.7 for the S-enantiomer.]
Suitability requirements
Resolution: NLT 2.9 between the S-enantiomer and montelukast, System suitability solution
Signal-to-noise ratio: NLT 10 for the montelukast peak, Sensitivity solution
Analysis
Sample: Sample solution
Calculate the percentage of S-enantiomer in the portion of Montelukast Sodium taken:
Result = (rU/rT) x 100
rU = peak response of the S-enantiomer from the Sample solution
rT = sum of the peak responses of the S-enantiomer and montelukast from the Sample solution
Acceptance criteria: NMT 0.2% of the S-enantiomer
5 SPECIFIC TESTS
WATER DETERMINATION, Method la(921): NMT 4.0%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers, protected from light. Store at room temperature.
USP REFERENCE STANDARDS (11)
USP Montelukast Sodium RS
USP Montelukast Dicyclohexylamine RS C35H36CINO3S · C12H23N 767.50
USP Montelukast Racernate RS
USP Montelukast for Peak Identification RS
(montelukast containing sulfoxide impurity, michael adducts 1 and 2, methylketone impurity, and methylstyrene impurity)


