Molindone Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
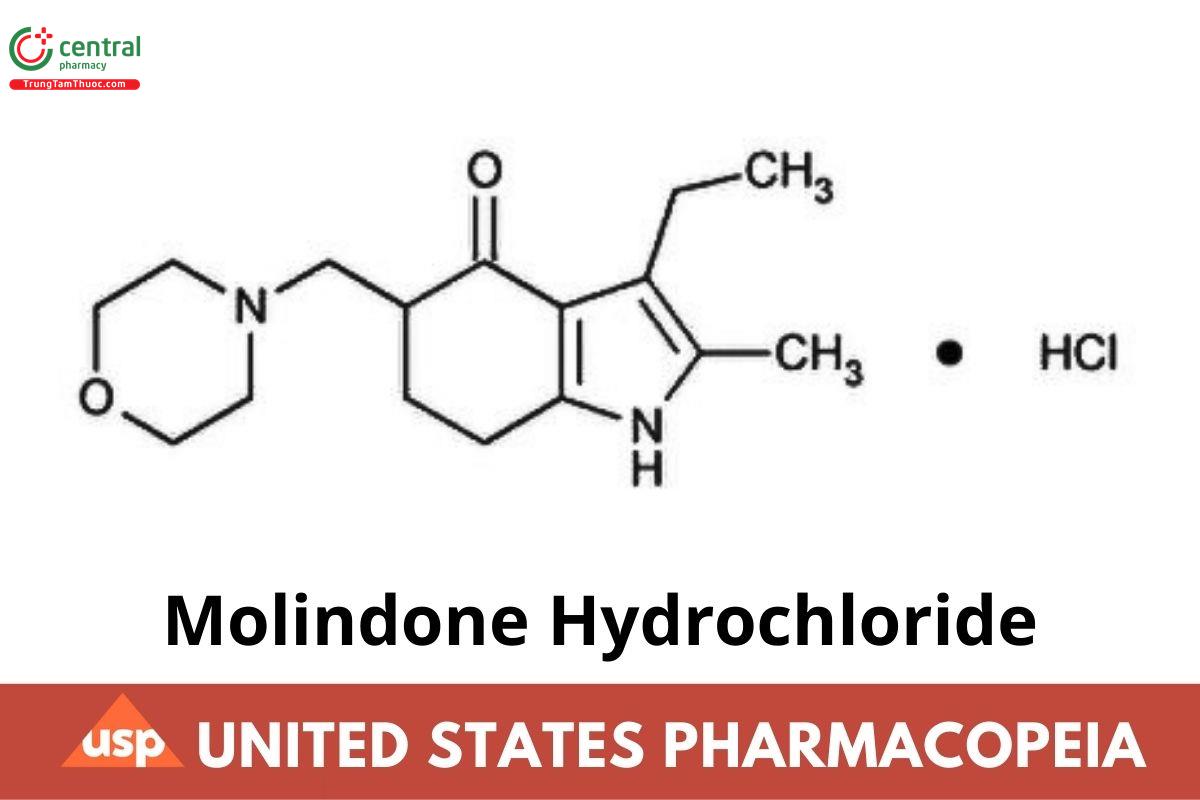
C₁₆H₂₄N₂O₂ · HCl 312.84
4H-Indol-4-one, 3-ethyl-1,5,6,7-tetrahydro-2-methyl-5-(4-morpholinylmethyl)-, monohydrochloride;
3-Ethyl-6,7-dihydro-2-methyl-5-(morpholinomethyl)indol-4(5H)-one monohydrochloride CAS RN®: 15622-65-8 UNII: 1DWS68PNE6
1 DEFINITION
Molindone Hydrochloride contains NLT 98.0% and NMT 101.5% of molindone hydrochloride (C₁₆H₂₄N₂O₂ · HCl), calculated on the anhydrous basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K. Do not dry the Standard or Sample.
Change to read:
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
C. Identification Tests-General 〈191〉, Chemical Identification Tests, Chloride: Meets the requirements
3 ASSAY
Change to read:
3.1 Procedure
Mobile phase: Dissolve 1.1 g of octanesulfonic acid sodium salt in 600 mL of water. Add 400 mL of methanol, 1 mL of glacial acetic acid, and 0.5 mL of triethylamine.
Diluent: Methanol and 0.01 N hydrochloric acid TS (40:60)
Standard solution: 0.5 mg/mL of USP Molindone Hydrochloride RS in Diluent
Sample solution: 0.5 mg/mL of Molindone Hydrochloride in Diluent
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: UV 254 nm
- Column: 4.6-mm × 25-cm; 5-µm packing L11
- Temperatures
- Autosampler: 4°
- Column: 35°
- Flow rate: 1.5 mL/min
- Injection volume: 5 µL
- Run time: NLT 1.7 times the retention time of molindone
System suitability
- Sample: Standard solution
- Suitability requirements
- Tailing factor: NMT 2.0
- Relative standard deviation: NMT 0.73%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of molindone hydrochloride (C₁₆H₂₄N₂O₂ · HCl) in the portion of Molindone Hydrochloride taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × 100
rᵤ = peak response of molindone from the Sample solution
rₛ = peak response of molindone from the Standard solution
Cₛ = concentration of USP Molindone Hydrochloride RS in the Standard solution (mg/mL)
Cᵤ = concentration of Molindone Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–101.5% on the anhydrous basis
4 IMPURITIES
4.1 Residue on Ignition 〈281〉: NMT 0.25%
Change to read:
4.2 Organic Impurities
Mobile phase and Diluent: Prepare as directed in the Assay.
System suitability solution: 2 mg/mL of USP Molindone Hydrochloride RS and 0.01 mg/mL of USP Molindone Related Compound A RS in Diluent
Sensitivity solution: 0.001 mg/mL of USP Molindone Hydrochloride RS in Diluent
Standard solution: 0.002 mg/mL each of USP Molindone Hydrochloride RS and USP Molindone Related Compound A RS in Diluent
Sample solution: 2 mg/mL of Molindone Hydrochloride in Diluent
Chromatographic system: Proceed as directed in the Assay, except for the Injection volume.
Injection volume: 20 µL
System suitability
Samples: System suitability solution, Standard solution, and Sensitivity solution
Suitability requirements
Resolution: NLT 5 between molindone and molindone related compound A, System suitability solution
Relative standard deviation: NMT 5.0% each of molindone and molindone related compound A, Standard solution
Signal-to-noise ratio: NLT 10 of molindone, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of molindone related compound A in the portion of Molindone Hydrochloride taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × 100
rᵤ = peak response of molindone related compound A from the Sample solution
rₛ = peak response of molindone related compound A from the Standard solution
Cₛ = concentration of USP Molindone Related Compound A RS in the Standard solution (mg/mL)
Cᵤ = concentration of Molindone Hydrochloride in the Sample solution (mg/mL)
Calculate the percentage of any unspecified impurity in the portion of Molindone Hydrochloride taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × 100
rᵤ = peak response of any unspecified impurity from the Sample solution
rₛ = peak response of molindone from the Standard solution
Cₛ = concentration of USP Molindone Hydrochloride RS in the Standard solution (mg/mL)
Cᵤ = concentration of Molindone Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: See Table 1.
The reporting threshold is 0.05%.
Table 1
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Molindone related compound A | 0.55 | 0.10 |
| Molindone | 1.0 | - |
| Any unspecified impurity | - | 0.10 |
| Total impurities | - | 0.50 |
5 SPECIFIC TESTS
Change to read:
5.1 pH 〈791〉
Sample solution: 10 mg/mL in water
Acceptance criteria: 4.0–5.0
5.2 Water Determination 〈921〉, Method I
NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers.
Change to read:
USP Reference Standards 〈11〉
USP Molindone Hydrochloride RS
USP Molindone Related Compound A RS
3-Ethyl-2-methyl-1,5,6,7-tetrahydro-4H-indol-4-one.
C₁₁H₁₅NO 177.25


