Mitoxantrone Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
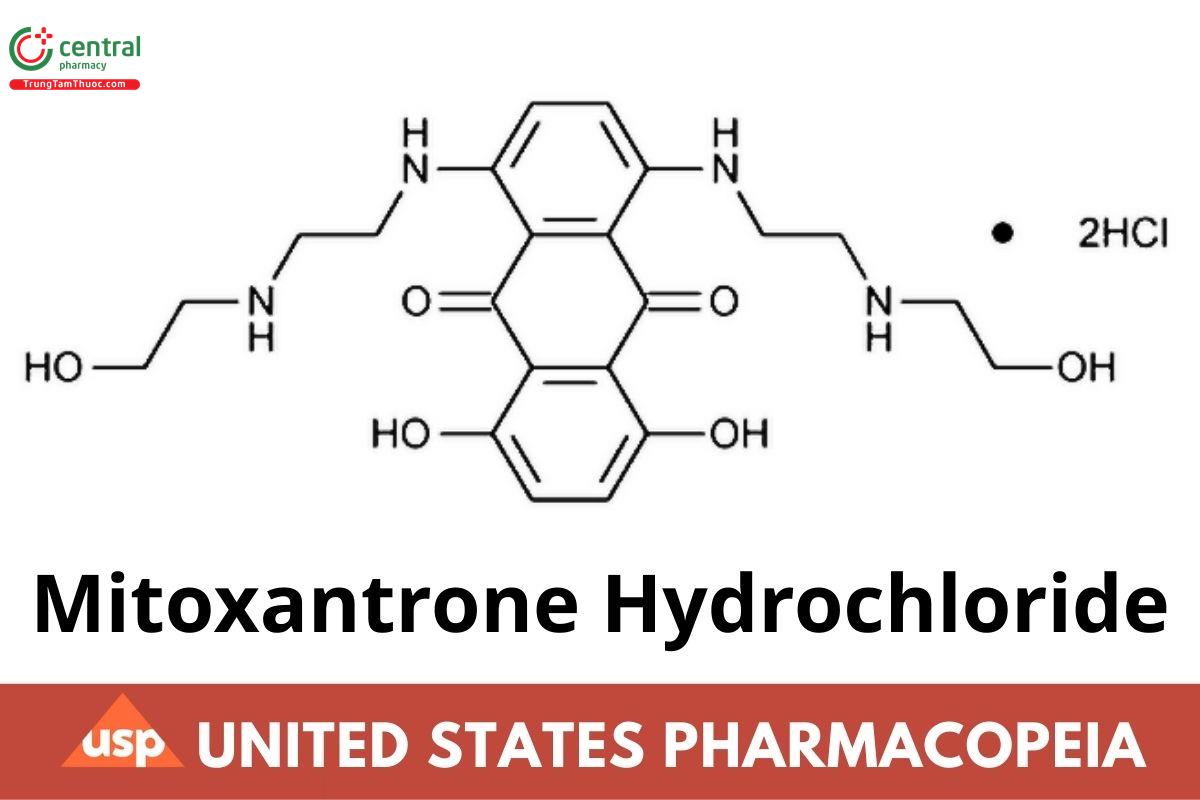
C22H28N4O6 · 2HCl 517.40
9,10-Anthracenedione, 1,4-dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]-, dihydrochloride; 1,4-Dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]anthraquinone dihydrochloride
CAS RN: 70476-82-3.
1 DEFINITION
Mitoxantrone Hydrochloride contains NLT 97.0% and NMT 102.0% of mitoxantrone hydrochloride (C22H28N4O6 · 2HCl), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy, 197A or 197K (CN 1-MAY-2020): Meets the requirements
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Solution A: 0.09 g/mL of sodium 1-heptanesulfonate prepared as follows. Dissolve 22.0 g of sodium 1-heptanesulfonate in 150 mL of water. Pass through a filter of NMT 0.5-µm pore size and transfer the filtrate to a 250-mL volumetric flask. Wash the filter with 50 mL of water and add the washings to the flask. Add 32.0 mL of glacial acetic acid to the flask, and dilute with water to volume.
Mobile phase: Acetonitrile, Solution A, and water (10:1:30)
System suitability solution: 0.2 mg/mL of monoalkyl mitoxantrone hydrochloride and 0.1 mg/mL of mitoxantrone hydrochloride from USP Mitoxantrone System Suitability Mixture RS in Mobile phase
Standard solution: 0.4 mg/mL of USP Mitoxantrone Hydrochloride RS prepared as follows. Transfer a suitable amount of USP Mitoxantrone Hydrochloride RS to an appropriate volumetric flask. Add 80% of the flask volume of Mobile phase. Sonicate for about 5 min to dissolve. Cool to room temperature and dilute with Mobile phase to volume.
Sample solution: 0.4 mg/mL of Mitoxantrone Hydrochloride prepared as follows. Transfer a suitable amount of Mitoxantrone Hydrochloride to an appropriate volumetric flask. Add 80% of the flask volume of Mobile phase. Sonicate for about 5 min to dissolve. Cool to room temperature and dilute with Mobile phase to volume.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 3.9-mm x 30-cm; 10-µm packing L11
[NOTE-After use, wash the column with a mixture of acetonitrile and water (50:50), and store in this mixture.]
Flow rate: 3 mL/min
Injection volume: 50 µL
System suitability
Samples: System suitability solution and Standard solution
[NOTE-The relative retention times for mitoxantrone and monoalkyl mitoxantrone hydrochloride are about 1.0 and 1.4, respectively.]
Suitability requirements
Resolution: NLT 3.0 between mitoxantrone and monoalkyl mitoxantrone hydrochloride, System suitability solution
Tailing factor: NMT 2.0 for mitoxantrone, System suitability solution
Capacity factor, k': NLT 3.5 for mitoxantrone, Standard solution
Relative standard deviation: NMT 0.73%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of mitoxantrone dihydrochloride (C22H28N4O6 · 2HCl) in the portion of Mitoxantrone Hydrochloride taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak area of mitoxantrone from the Sample solution
rs = peak area of mitoxantrone from the Standard solution
Cs = concentration of USP Mitoxantrone Hydrochloride RS in the Standard solution (mg/mL)
Cu = concentration of Mitoxantrone Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–102.0% on the anhydrous basis
4 IMPURITIES
4.1 ORGANIC IMPURITIES
Analysis: Using the chromatogram of the Sample solution obtained as directed in the Assay, calculate the percentage of each impurity in the portion of Mitoxantrone Hydrochloride taken:
Result = (rU /rT ) × 100
rU = peak response of each impurity
rT = sum of all peak responses
Acceptance criteria
Individual impurities: NMT 1.0%
Total impurities: NMT 2.0%
4.2 LIMIT OF ALCOHOL
Proceed as directed in Residual Solvents (467).
Acceptance criteria: NMT 1.5%
5 SPECIFIC TESTS
WATER DETERMINATION (921), Method 1: NMT 6.0%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers.
USP REFERENCE STANDARDS (11)
USP Mitoxantrone Hydrochloride RS
USP Mitoxantrone System Suitability Mixture RS
[(2-hydroxyethyl) 0.2 mg of 1-Amino-5,8-dihydroxy-4-((2-[(2-hydroxyethyl)amino]ethyl)amino) anthracene-9,10-dione hydrochloride [monoalkyl mitoxantrone hydrochloride (C18H19N3O5 · HCI, 393.82)) and 0.1 mg of USP Mitoxantrone Hydrochloride RS


