Mirtazapine
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
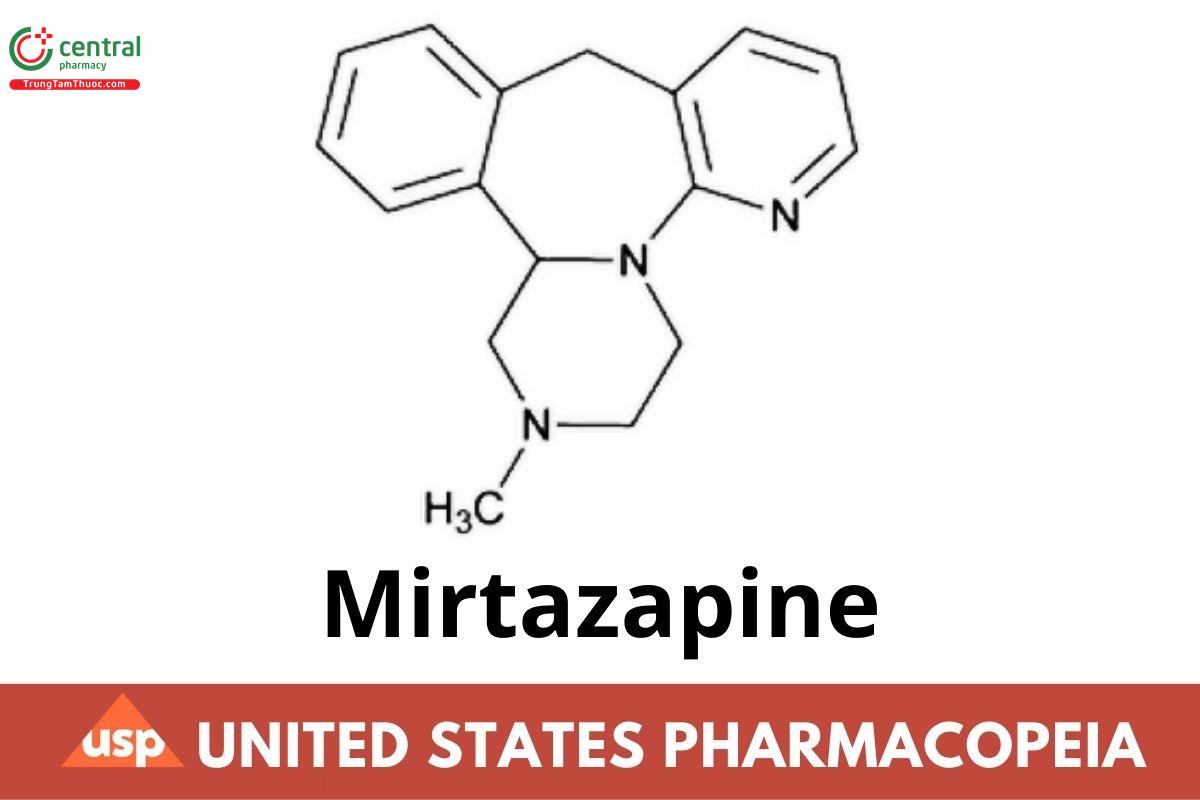
C17H19N3 265.35
Pyrazino[2,1-a]pyrido[2,3-c][2]benzazepine,1,2,3,4,10,14b-hexahydro-2-methyl-;1,2,3,4,10,14b-Hexahydro-2-methylpyrazino[2,1-a]pyrido[2,3-c][2]-benzazepine CAS RN: 85650-52-8; UNII: A051Q2099Q.
1 DEFINITION
Mirtazapine contains NLT 98.0% and NMT 102.0% of mirtazapine (C17H19N3), calculated on the anhydrous basis.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Standard solution: 0.3 mg/mL of USP Mirtazapine RS in Diluent OFFICIAL
Diluent: Acetonitrile and water (1:1)
Buffer : Dissolve 18 g of tetramethylammonium hydroxide pentahydrate in 950 mL of water. Adjust with phosphoric acid to a pH of 7.4. Dilute with water to 1 L.
12.5:7.5:65) Mobile phase: Acetonitrile, methanol, tetrahydrofuran, and Buffer (15:12.5:7.5:65)
Sample solution: 0.3 mg/mL of Mirtazapine in Diluent
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 290 nm
Column: 4.6-mm x 25-cm; packing L1
Column temperature: 40°
Flow rate: 1.5 mL/min
Injection volume: 10 µL
System suitability
Sample: Standard solution
Suitability requirements
Column efficiency: NLT 7000 theoretical plates
Tailing factor: NMT 2.0
Relative standard deviation: NMT 1.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of mirtazapine (C17H19N3) in the portion of Mirtazapine taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of USP Mirtazapine RS in the Standard solution (mg/mL)
Cu = concentration of Mirtazapine in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the anhydrous basis
4 IMPURITIES
RESIDUE ON IGNITION (281); NMT 0.1%
ORGANIC IMPURITIES
Diluent, Buffer, and Mobile phase: Prepare as directed in the Assay.
System suitability solution: 1.5 mg/mL of USP Mirtazapine Resolution Mixture RS in Diluent
Standard solution: 0.0015 mg/mL of USP Mirtazapine RS in Diluent
Sample solution: 1.5 mg/mL of Mirtazapine in Diluent
Chromatographic system
(See Chromatography (621) System Suitability.)
Mode: LC
Detector: UV 240 nm
Column: 4.6-mm x 25-cm; packing L1
Column temperature: 40°
Flow rate: 1.5 mL/min
Injection volume: 10 µL
Run time: Twice the retention time of mirtazapine
System suitability
Samples: System suitability solution and Standard solution
[NOTE-The relative retention times are listed in Table 1.1
Suitability requirements
Resolution: NLT 1.5 between acyclomirtazapine methyl derivative (impurity E) and 10-ketomirtazapine (impurity F), System suitability solution
Tailing factor: NMT 2.0, Standard solution
Relative standard deviation: NMT 10.0%, Standard solution.
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Mirtazapine taken:
Result = (ru /rs ) × (Cs /Cu ) × (1/F) × 100
ru = peak response of any impurity from the Sample solution
rs = peak response of mirtazapine from the Standard solution
Cs = concentration of USP Mirtazapine RS in the Standard solution (mg/mL)
Cu = concentration of Mirtazapine in the Sample solution (mg/mL)
F = relative response factor (see Table 1)
[Note—Disregard any peak with a result of 0.05% or less, as calculated using the formula given above.]
Acceptance criteria: See Table 1.
Table 1
| Impurity Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Mirtazapine N-oxidea | 0.2 | 0.8 | 0.1 |
| Acyclomirtazapine alcoholb | 0.3 | 0.8 | 0.1 |
| 1-Ketomirtazapinec | 0.35 | 1.0 | 0.1 |
| Desmethylmirtazapined | 0.4 | 1.0 | 0.1 |
| Mirtazapine | 1.0 | - | - |
| Acyclomirtazapine methyl derivativee | 1.3 | 1.0 | 0.1 |
| 10-Ketomirtazapinef | 1.35 | 5.0 | 0.1 |
| Any individual unspecied impurity | - | 1.0 | 0.10 |
| Total impurities | - | - | 0.5 |
[Note—Disregard any peak representing less than 0.05% of the main peak and any peak that is due to the Diluent.]
a 1,2,3,4,10,14b-Hexahydro-2-methylpyrazino[2,1-a]pyrido[2,3-c][2]benzazepine 2-oxide (Impurity A).
b (2-(4-Methyl-2-phenylpiperazin-1-yl)pyridin-3-yl)methanol (Impurity B).
c (2-Methyl-3,4,10,14b-tetrahydrobenzo[c]pyrazino[1,2-a]pyrido[3,2-f]azepin-1(2H)-one (Impurity C).
d 1,2,3,4,10,14b-Hexahydropyrazino[2,1-a]pyrido[2,3-c][2]benzazepine (Impurity D).
e 4-Methyl-1-(3-methylpyridin-2-yl)-2-phenylpiperazine (Impurity E).
f 2-Methyl-1,2,3,4-tetrahydrobenzo[c]pyrazino[1,2-a]pyrido[3,2-f]azepin-10(14bH)-one (Impurity F).
5 SPECIFIC TESTS
Water Determination, Method I 〈921〉: NMT 3.5%
Optical Rotation, Specific Rotation 〈781S〉
Sample solution: 10 mg/mL, in denatured alcohol
Acceptance criteria: +2° to −2°
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers, and store at controlled room temperature.
Labeling: Label it to indicate whether it is anhydrous or hemihydrate.
Change to read:
USP Reference Standards 〈11〉
USP Mirtazapine RS
USP Mirtazapine Resolution Mixture RS
Mirtazapine.
Impurity A: 1,2,3,4,10,14b-Hexahydro-2-methylpyrazino[2,1-a]pyrido[2,3-c][2]benzazepine 2-oxide.
Impurity B: (2-(4-Methyl-2-phenylpiperazin-1-yl)pyridin-3-yl)methanol.
Impurity C: (2-Methyl-3,4,10,14b-tetrahydrobenzo[c]pyrazino[1,2-a]pyrido[3,2-f]azepin-1(2H)-one.
Impurity D: [Note—This impurity may be available either as the free base form or as the hydrochloride salt form.] (ERR 1-Apr-2021) 1,2,3,4,10,14b-Hexahydropyrazino[2,1-a]pyrido[2,3-c][2]benzazepine or 1,2,3,4,10,14b-Hexahydropyrazino[2,1-a]pyrido[2,3-c][2]benzazepine hydrochloride. (ERR 1-Apr-2021)
Impurity E: 4-Methyl-1-(3-methylpyridin-2-yl)-2-phenylpiperazine.
Impurity F: 2-Methyl-1,2,3,4-tetrahydrobenzo[c]pyrazino[1,2-a]pyrido[3,2-f]azepin-10(14bH)-one.


