Minocycline Periodontal System
If you find any inaccurate information, please let us know by providing your feedback here

Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Minocycline Periodontal System is an extended-release formulation of Minocycline Hydrochloride containing the equivalent of NLT 90.0% and NMT 120.0% of the labeled amount of minocycline (C23H27N3O7)
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Ultraviolet-Visible Spectroscopy: 197U (CN 1-May-2020)
Wavelength range: 250-450 nm
Standard stock solution: Transfer USP Minocycline Hydrochloride RS to a suitable volumetric flask. Dissolve first in dimethylformamide, using about 20% of the final volume, then dilute with water to volume, and mix to obtain a solution having a known concentration of about 0.48 mg/mL of minocycline.
Standard solution: 0.024 mg/mL minocycline hydrochloride from Standard stock solution in water
Sample stock solution: Transfer Minocycline Periodontal System equivalent to 12 mg of minocycline hydrochloride to a 25-mL volumetric flask. Add 5.0 mL of dimethylformamide, and mix to dissolve. Dilute with water to volume and filter.
Sample solution: 0.024 mg/mL minocycline hydrochloride from Sample stock solution in water
Acceptance criteria: The Sample solution exhibits maxima at the same wavelengths as the Standard solution, concomitantly measured.
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Mobile phase: Dimethylformamide, 0.2 M ammonium oxalate, and 0.1 M edetate disodium (25:55:20), adjusted with 0.4 M aqueous tetrabutylammonium hydroxide solution to a pH of 6.3 ± 0.2
Diluent: Dimethylformamide and methanol (1:1)
System suitability solution: Prepare a solution in water containing 2 mg/mL USP Minocycline Hydrochloride RS. Heat over a steam bath for 60 min. To one part of this solution add four parts of Mobile phase and mix. Refrigerate the solution immediately after preparation and during analysis, using a refrigerated autosampler.
Standard solution: 0.4 mg/mL of minocycline from USP Minocycline Hydrochloride RS in Diluent. Refrigerate the solution immediately after preparation and during analysis, using a refrigerated autosampler. [NOTE-Use low-actinic glassware.]
Sample solution: Mix the contents of NLT 10 dispensing units of Minocycline Periodontal System. Transfer a portion of the mixture, equivalent to 10 mg of minocycline, into a 25-mL volumetric flask. Add Diluent and sonicate for 2-5 min, or until the sample is dissolved. Dilute with Diluent to volume, and mix to obtain a solution having a nominal concentration of 0.4 mg/mL of minocycline, based on the label claim. Refrigerate the solution immediately after preparation and during analysis, using a refrigerated autosampler. [NOTE-Use low-actinic glassware.]
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm
Guard column: 4.6-mm x 3-cm; 10-µm packing L.7
Column: 4.6-mm x 15-cm; 5-µm packing L7
Flow rate: 2 mL/min
Autosampler temperature: 5o
Injection size: 10 µL
System suitability
Samples: System suitability solution and Standard solution
[NOTE-The relative retention times of epiminocycline and minocycline are 0.81 and 1, respectively.]
Suitability requirements
Resolution: NLT 2.0 between epiminocycline and minocycline, System suitability solution
Tailing factor: NMT 2.0 for the minocycline peak, System suitability solution
Relative standard deviation: NMT 2.0% for the minocycline peak, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of minocycline (C23H27N3O7) in the portion of the Minocycline Periodontal System taken:
Result = (ru /rs ) × (Cs /Cu ) × P × F × 100
ru = peak response of minocycline from the Sample solution
rs = peak response of minocycline from the Standard solution
Cs = concentration of USP Minocycline Hydrochloride RS in the Standard solution (mg/mL)
Cu = nominal concentration of minocycline in the Sample solution (mg/mL)
P = potency of minocycline in USP Minocycline Hydrochloride RS (µg/mg)
F = conversion factor, 0.001 mg/µg
Acceptance criteria: 90.0%–120.0%
4 PERFORMANCE TESTS
DISSOLUTION
Medium: 6.9 g/L of monobasic sodium phosphate monohydrate in water, adjusted with phosphoric acid to a pH of 4.2. This solution is stable for 10 days.
Apparatus: Tube rotator. [NOTE-Suitable equipment is available as Labquake® tube rotator, catalog number 400110.]
0.1 M Edetate disodium: 37.2 g/L of edetate disodium in water
0.2 M Ammonium oxalate: 28.4 g/L of ammonium oxalate in water
Mobile phase: Mix 310 mL of 0.1 M Edetate disodium and 500 mL of 0.2 M Ammonium oxalate, adjust with 0.4 M aqueous tetrabutylammonium hydroxide to a pH of 6.2, and add 175 mL of dimethylformamide. The injector wash solution is a mixture of dimethylformamide and water (25:75).
Standard stock solution: 0.11 mg/mL of USP Minocycline Hydrochloride RS in Medium
Standard solutions: Dilute the Standard stock solution with Medium to obtain solutions with final concentrations of 0.088 mg/mL, 0.0528 mg/mL, 0.0352 mg/mL, 0.022 mg/mL, and 0.0176 mg/mL.
System suitability solution: Transfer 10 mg of USP Minocycline Hydrochloride RS to a 50-mL beaker. Add 5 mL of water and heat on a steam bath for 60 min. Add 20 mL of Medium or Mobile phase, and mix well. Store at 5o
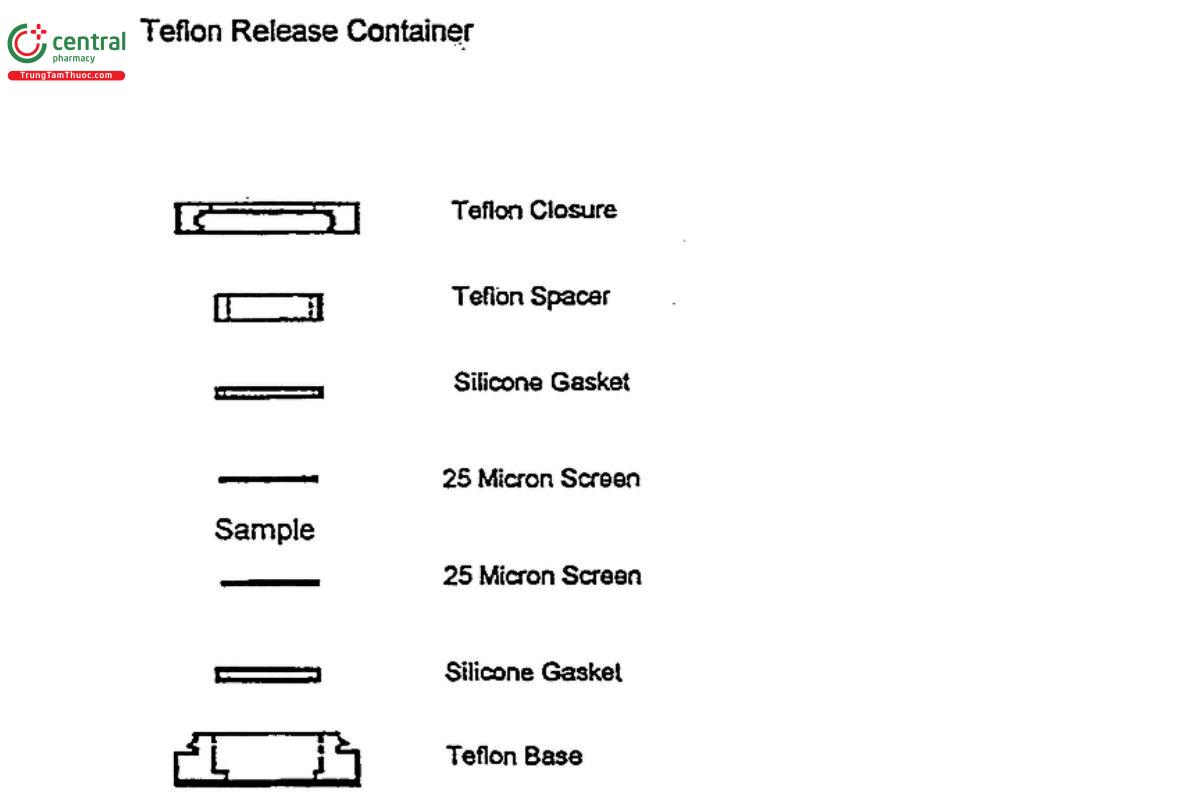
Sample solution: Use borosilicate glass tubes, 25 mm outside diameter and 15 cm long. Close the tubes with a snap type cell with a Teflon prong consisting of a Teflon closure and holder that snap together, two 25-um stainless steel screens, two silicone gaskets, and a Teflon spacer (see Figure 1). Prepare six tubes as follows: partially assemble a release tube and tare its weight; dispense one dose of Minocycline Periodontal System into a partially assembled release cell (see Figure 1); record the sample weight in mg; assemble the cell so that the sample is enclosed between the two 25-um screens; close the cells and place each one of them into separate glass tubes containing 10 mL of Medium previously equilibrated at 37o; add the Teflon prong, and cap the tube with Teflon faced rubber-lined caps; seal with Teflon tape. Place the tubes in the tube rotator. Place the tube rotator in a convection incubator that is maintained at 37o. Allow the tubes to rotate for 4 h. Remove the solution under test, and add 10 ml of Medium previously equilibrated at 37o, Replace the tubes in the apparatus and rotate for 20 h (24 h total). Repeat the sampling procedure after 24 h (48 h total), and after another 24 h (72 h total).
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 280 nm
Guard column: 4.6-mm × 3-cm; 10-µm packing L7
Column: 4.6-mm × 3.3-cm; 5-µm packing L1
Flow rate: 1.5 mL/min
Autosampler temperature: 5°
Injection size: 20 µL for the 4 and 24 h time points; 50 µL for the 48 and 72 h time points
Suitability requirements
Samples: System suitability solution and Standard solutions
Resolution: NLT 2.0 between epiminocycline and minocycline. Inject 20 µL of the System suitability solution.
Tailing factor: NMT 2.0. Inject 20 µL of the System suitability solution.
Relative standard deviation: NMT 2.0% for the minocycline peak, any of the Standard solutions
Analysis: Construct a calibration curve for each sampling interval by plotting the concentration of the Standard solutions versus peak area.
Calculate the slopes and y-intercepts using linear regression analysis.
Calculate the release rate of minocycline:
Resulti = [(rUi − yi )/Si ] × 10/( i × W × A)
i = sampling time, 4, 24, 48, 72 h
rUi = peak response from each of the Standard solutions at time i
yi = y-intercept of the calibration curve at sampling time i
Si = slope of the calibration curve at sampling time i
W = weight of the sample (mg)
A = amount of minocycline in the sample (mg/mg of sample) as determined in the Assay
Tolerances
| Time (h) | Release Rate (µg/h) Average of 6 Measurements |
| 0–4 | NLT 25 |
| 4–24 | NLT 1.0 |
| 24–48 | NLT 0.2 |
| 48–72 | NLT 0.05 |
UNIFORMITY OF DOSAGE UNITS (905): Meet the requirements
5 IMPURITIES
ORGANIC IMPURITIES
PROCEDURE
Mobile phase, Diluent, System suitability solution, Standard solution, Sample solution, Chromatographic system, and System
suitability: Proceed as directed in the Assay.
Analysis
Sample: Sample solution
Calculate the percentage of each related compound in the portion of Minocycline Periodontal System taken:
Result = (rU /rT ) × 100
rU = peak response of each impurity from the Sample solution
rT = sum of the peak responses from the Sample solution. [Note—Exclude peaks eluting in the solvent front.]
Acceptance criteria
Individual impurities: NMT 6.0% of epiminocycline
Total impurities (excluding epiminocycline): NMT 3.5%
6 SPECIFIC TESTS
WATER DETERMINATION, Method (921): NMT 5.0%
MICROBIUL ENUMERATION TESTS (61)and TESTS FOR SPECIFIED ORGANISME (62); The total aerobic microbial count does not exceed 1000 cfu/g, the total combined molds and yeasts count does not exceed 100 cfu/g; and the product meets the requirements of the test for the absence of Escherichia coll
7 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in a tight, light-resistant container. Store at controlled room temperature.
USP REFERENCE STANDARDS (11)
SP Minocoline Hedrochloride RS


